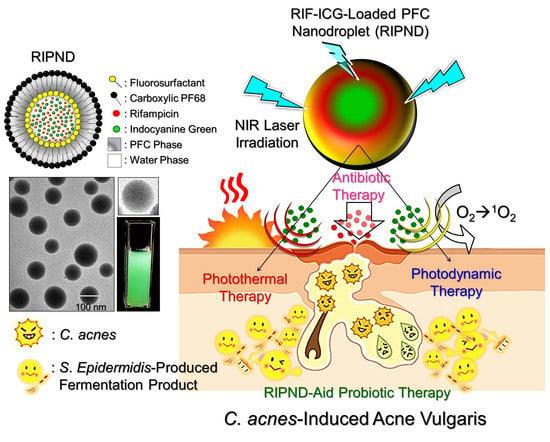
Novel Rifampicin and Indocyanine Green Co-Loaded Perfluorocarbon Nanodroplets Provide Effective In Vivo Photo–Chemo–Probiotic Antimicrobility against Pathogen of Acne Vulgaris Cutibacterium acnes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication and Characterization of the RIPNDs
2.2. Microbial Cultivation
2.3. Measurement of Antimicrobial Efficacy of the RIPNDs in vitro
2.4. Cell Culture
2.5. In Vitro Cytotoxicity Assay
2.6. Animal Study
2.7. Evaluation of Antimicrobial Effect of the RIPNDs In Vivo
2.8. Measurement of Inflammatory Response In Vivo
2.9. Histological Study
2.10. Statistical Analysis
3. Results and Discussion
3.1. Morphological and Physicochemical Analyses of the RIPNDs
3.2. Antibacterial Effect of the RIPNDs In Vitro
3.3. Cytotoxicity of the FPM and RIPNDs In Vitro
3.4. Anti-Inflammatory Response of the RIPNDs In Vivo
3.5. Antimicrobial Effect of the RIPNDs In Vivo
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AV | Acne vulgaris |
| CFU | Colony forming units |
| Ffar1 | Free fatty acid receptor 1 |
| FPM | Fermentation product medium |
| GPR40 | G protein-coupled receptor 40 |
| HDAC | Histone deacetylase |
| H&E | Hematoxylin and eosin |
| ICG | Indocyanine green |
| MBC | Minimal bactericidal concentration |
| MIP-2 | Macrophage inflammatory protein-2 |
| MPI | Microbial population index |
| NIR | Near infrared |
| OD | Optical density |
| PFC | Perfluorocarbon |
| PFOB | Perfluorooctyl bromide |
| RCM | Reinforced clostridium medium |
| RIF | Rifampicin |
| RIPND | Rifampicin-indocyanine green-loaded perfluorocarbon nanodroplets |
References
- Walsh, T.R.; Efthimiou, J.; Dréno, B. Systematic review of antibiotic resistance in acne: An increasing topical and oral threat. Lancet Infect. Dis. 2016, 16, e23–e33. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.J.; Van Dyke, G.S.; Kim, J. Update on pathogenesis and treatment of acne. Curr. Opin. Pediatr. 2003, 15, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.; Vu, H.L.; Mariwalla, K.; Saedi, N. Acne Scarring-Pathogenesis, Evaluation, and Treatment Options. J. Clin. Aesthet. Dermatol. 2017, 10, 12–23. [Google Scholar] [PubMed]
- Garg, T. Current nanotechnological approaches for an effective delivery of bio-active drug molecules in the treatment of acne. Artif. Cells Nanomed. Biotechnol. 2016, 44, 98–105. [Google Scholar] [CrossRef]
- Ochsendorf, F. Systemic antibiotic therapy of acne vulgaris. J. Dtsch. Dermatol. Ges. 2006, 4, 828–841. [Google Scholar] [CrossRef]
- Levine, R.M.; Rasmussen, J.E. Intralesional corticosteroids in the treatment of nodulocystic acne. Arch. Dermatol. 1983, 119, 480–481. [Google Scholar] [CrossRef]
- Sagransky, M.; Yentzer, B.A.; Feldman, S.R. Benzoyl peroxide: A review of its current use in the treatment of acne vulgaris. Expert Opin. Pharmacother. 2009, 10, 2555–2562. [Google Scholar] [CrossRef]
- Kunimoto, D.; Warman, A.; Beckon, A.; Doering, D.; Melenka, L. Severe hepatotoxicity associated with rifampin-pyrazinamide preventative therapy requiring transplantation in an individual at low risk for hepatotoxicity. Clin. Infect. Dis. 2003, 36, e158–e161. [Google Scholar] [CrossRef] [Green Version]
- Ronald, L.A.; FitzGerald, J.M.; Bartlett-Esquilant, G.; Schwartzman, K.; Benedetti, A.; Boivin, J.F.; Menzies, D. Treatment with Isoniazid or Rifampin for Latent Tuberculosis Infection: Population-Based Study of Hepatotoxicity, Completion, and Costs. Eur. Respir. J. 2020, 55, 1902048. [Google Scholar] [CrossRef]
- Layton, A.M.; Dreno, B.; Gollnick, H.P.; Zouboulis, C.C. A review of the European Directive for prescribing systemic isotretinoin for acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 773–776. [Google Scholar] [CrossRef]
- Wang, X.; Tian, Y.; Liao, X.; Tang, Y.; Ni, Q.; Sun, J.; Zhao, Y.; Zhang, J.; Teng, Z.; Lu, G. Enhancing selective photosensitizer accumulation and oxygen supply for high-efficacy photodynamic therapy toward glioma by 5-aminolevulinic acid loaded nanoplatform. J. Colloid. Interface Sci. 2020, 565, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Quéreux, G.; Brocard, A.; Saint-Jean, M.; Peuvrel, L.; Knol, A.C.; Allix, R.; Khammari, A.; Renaut, J.J.; Dréno, B. Photodynamic therapy with methyl-aminolevulinic acid for paucilesional mycosis fungoides: a prospective open study and review of the literature. J. Am. Acad. Dermatol. 2013, 69, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Grandi, V.; Baldi, I.; Cappugi, P.; Mori, M.; Pimpinelli, N. Indole 3-acetic acid-photodynamic therapy in the treatment of multiple actinic keratoses: A proof of concept pilot study. Photodiagnosis Photodyn. Ther. 2016, 16, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Lin, Y.C. Anti-EGFR Indocyanine Green-Mitomycin C-loaded Perfluorocarbon Double Nanoemulsion: A Novel Nanostructure for Targeted Photochemotherapy of Bladder Cancer Cells. Nanomaterials 2018, 8, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, M.S.; Yun, S.J.; Beom, H.J.; Park, H.R.; Lee, J.B. Comparative study of the bactericidal effects of 5-aminolevulinic acid with blue and red light on Propionibacterium acnes. J. Dermatol. 2011, 38, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Lekakh, O.; Mahoney, A.M.; Novice, K.; Kamalpour, J.; Sadeghian, A.; Mondo, D.; Kalnicky, C.; Guo, R.; Peterson, A.; Tung, R. Treatment of Acne Vulgaris With Salicylic Acid Chemical Peel and Pulsed Dye Laser: A Split Face, Rater-Blinded, Randomized Controlled Trial. J. Lasers Med. Sci. 2015, 6, 167–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, J.M. Combined 400-600nm and 800-1200nm Intense Pulsed Phototherapy of Facial Acne Vulgaris. J. Drugs Dermatol. 2019, 18, 1116–1122. [Google Scholar]
- Das, S.; Reynolds, R.V. Recent advances in acne pathogenesis: implications for therapy. Am. J. Clin. Dermatol. 2014, 15, 479–488. [Google Scholar] [CrossRef]
- Partha, S.; Niyati, A.; Rohanb, L.; Srinivasanc, S. Acne vulgaris: An update on current therapy and advances in treatment strategies. Int. J. Pharm. Sci. Rev. Res. 2016, 40, 234–244. [Google Scholar]
- Saxena, V.; Sadoqi, M.; Shao, J. Degradation kinetics of indocyanine green in aqueous solution. J. Pharm. Sci. 2003, 92, 2090–2097. [Google Scholar] [CrossRef]
- Cherrick, G.R.; Stein, S.W.; Leevy, C.M.; Davidson, C.S. Indocyanine green: Observations on its physical properties, plasma decay, and hepatic extraction. J. Clin. Investig. 1960, 39, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Desmettre, T.; Devoisselle, J.M.; Mordon, S. Fluorescence properties and metabolic features of indocyanine green (ICG) as related to angiography. Surv. Ophthalmol. 2000, 45, 15–27. [Google Scholar] [CrossRef]
- Mottin, V.H.M.; Suyenaga, E.S. An approach on the potential use of probiotics in the treatment of skin conditions: acne and atopic dermatitis. Int. J. Dermatol. 2018, 57, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, H.; Smoragiewicz, W. Role of probiotics in the prevention and treatment of meticillin-resistant Staphylococcus aureus infections. Int. J. Antimicrob. Agents 2013, 42, 475–481. [Google Scholar] [CrossRef]
- Lopes, E.G.; Moreira, D.A.; Gullón, P.; Gullón, B.; Cardelle-Cobas, A.; Tavaria, F.K. Topical application of probiotics in skin: adhesion, antimicrobial and antibiofilm in vitro assays. J. Appl. Microbiol. 2017, 122, 450–461. [Google Scholar] [CrossRef]
- Yu, Y.; Dunaway, S.; Champer, J.; Kim, J.; Alikhan, A. Changing our microbiome: Probiotics in dermatology. Br. J. Dermatol. 2020, 182, 39–46. [Google Scholar] [CrossRef]
- Shu, M.; Wang, Y.; Yu, J.; Kuo, S.; Coda, A.; Jiang, Y.; Gallo, R.L.; Huang, C.M. Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLoS ONE 2013, 8, e55380. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Kuo, S.; Shu, M.; Yu, J.; Huang, S.; Dai, A.; Two, A.; Gallo, R.L.; Huang, C.M. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: Implications of probiotics in acne vulgaris. Appl. Microbiol. Biotechnol. 2014, 98, 411–424. [Google Scholar] [CrossRef] [Green Version]
- Kao, M.S.; Wang, Y.; Marito, S.; Huang, S.; Lin, W.Z.; Gangoiti, J.A.; Barshop, B.A.; Hyun, C.; Lee, W.R.; Sanford, J.A.; et al. The mPEG-PCL Copolymer for Selective Fermentation of Staphylococcus lugdunensis Against Candida parapsilosis in the Human Microbiome. J. Microb. Biochem. Technol. 2016, 8, 259–265. [Google Scholar]
- Wang, Y.; Kao, M.S.; Yu, J.; Huang, S.; Marito, S.; Gallo, R.L.; Huang, C.M. A Precision Microbiome Approach Using Sucrose for Selective Augmentation of Staphylococcus epidermidis Fermentation against Propionibacterium acnes. Int. J. Mol. Sci. 2016, 17, 1870. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, K.H.; Huang, C.H.; Lee, Y.H. Development of Rifampicin-Indocyanine Green-Loaded Perfluorocarbon Nanodroplets for Photo-Chemo-Probiotic Antimicrobial Therapy. Front. Pharmacol. 2018, 9, 1254. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.F.; Nakatsuji, T.; Zhu, W.; Gallo, R.L.; Huang, C.M. Passive immunoprotection targeting a secreted CAMP factor of Propionibacterium acnes as a novel immunotherapeutic for acne vulgaris. Vaccine 2011, 29, 3230–3238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kligman, A.M. An overview of acne. J. Investig. Dermatol. 1974, 62, 268–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, F.; Serpa, J.; Domingues, G.; Silva, G.; Almeida, A.; Félix, A. Cell death induced by HDACS inhibitors in ovarian cancer cell lines (serous and clear cells carcinomas)-Role of NOTCH, TP53 and FN1. BMC Proc. 2010, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Lee, E.J.; Lee, J.C.; Kim, W.K.; Kim, H.S. Anti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: involvement of NF-kappaB and ERK signaling pathways. Int. Immunopharmacol. 2007, 7, 70–77. [Google Scholar] [CrossRef]
- Lohman, R.J.; Iyer, A.; Fairlie, T.J.; Cotterell, A.; Gupta, P.; Reid, R.C.; Vesey, D.A.; Sweet, M.J.; Fairlie, D.P. Differential Anti-inflammatory Activity of HDAC Inhibitors in Human Macrophages and Rat Arthritis. J. Pharmacol. Exp. Ther. 2016, 356, 387–396. [Google Scholar] [CrossRef]
- Hara, T.; Hirasawa, A.; Ichimura, A.; Kimura, I.; Tsujimoto, G. Free fatty acid receptors FFAR1 and GPR120 as novel therapeutic targets for metabolic disorders. J. Pharm. Sci. 2011, 100, 3594–3601. [Google Scholar] [CrossRef]
- Moonwiriyakit, A.; Koval, M.; Muanprasat, C. Pharmacological stimulation of G-protein coupled receptor 40 alleviates cytokine-induced epithelial barrier disruption in airway epithelial Calu-3 cells. Int. Immunopharmacol. 2019, 73, 353–361. [Google Scholar] [CrossRef]
- Furustrand Tafin, U.; Corvec, S.; Betrisey, B.; Zimmerli, W.; Trampuz, A. Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob. Agents Chemother. 2012, 56, 1885–1891. [Google Scholar] [CrossRef] [Green Version]





| Dosage | NIR | FPM | [RIF]/[ICG] (μM) | |||||
|---|---|---|---|---|---|---|---|---|
| Treatment | #1 | #2 | #3 | #4 | #5 | #6 | ||
| *B | − | − | - | |||||
| + | − | |||||||
| − | + | |||||||
| *B + RIF | − | − | 0.24/- | 0.47/- | 0.95/- | 1.9/- | 3.8/- | 7.6/- |
| *B + RIPNDs | − | − | 0.24/1.25 | 0.47/2.5 | 0.95/5 | 1.9/10 | 3.8/20 | 7.6/40 |
| + | − | |||||||
| + | + | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, K.-H.; Huang, C.-M.; Lee, Y.-H. Novel Rifampicin and Indocyanine Green Co-Loaded Perfluorocarbon Nanodroplets Provide Effective In Vivo Photo–Chemo–Probiotic Antimicrobility against Pathogen of Acne Vulgaris Cutibacterium acnes. Nanomaterials 2020, 10, 1095. https://doi.org/10.3390/nano10061095
Hsiao K-H, Huang C-M, Lee Y-H. Novel Rifampicin and Indocyanine Green Co-Loaded Perfluorocarbon Nanodroplets Provide Effective In Vivo Photo–Chemo–Probiotic Antimicrobility against Pathogen of Acne Vulgaris Cutibacterium acnes. Nanomaterials. 2020; 10(6):1095. https://doi.org/10.3390/nano10061095
Chicago/Turabian StyleHsiao, Kuang-Hung, Chun-Ming Huang, and Yu-Hsiang Lee. 2020. "Novel Rifampicin and Indocyanine Green Co-Loaded Perfluorocarbon Nanodroplets Provide Effective In Vivo Photo–Chemo–Probiotic Antimicrobility against Pathogen of Acne Vulgaris Cutibacterium acnes" Nanomaterials 10, no. 6: 1095. https://doi.org/10.3390/nano10061095