Cellulose Nanocrystals/Chitosan-Based Nanosystems: Synthesis, Characterization, and Cellular Uptake on Breast Cancer Cells
Abstract
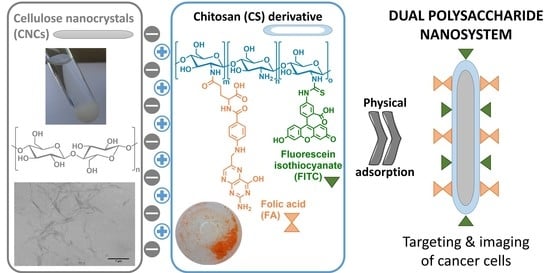
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Materials, and Cells
2.2. Preparation of Cellulose Nanocrystals (CNCs)
2.3. Synthesis of the FA-CS-FITC Derivative
2.4. Preparation of the CNC Nanosystems Functionalized with the FA-CS-FITC Derivative
2.5. Characterization Methods
2.6. Stability Tests
2.7. Cell Culture
2.8. In Vitro Cytotoxicity Assay
2.9. Cellular Uptake Assay
2.10. Cellular Exometabolomics
2.11. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of the CNCs/FA-CS-FITC Nanosystems
3.2. Cellular Viability
3.3. Cellular Internalization
3.4. Cellular Exometabolomics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vilela, C.; Pinto, R.J.B.; Pinto, S.; Marques, P.A.A.P.; Silvestre, A.J.D.; Freire, C.S.R. Polysaccharide Based Hybrid Materials: Metals and Metal Oxides, Graphene and Carbon Nanotubes, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-030-00346-3. [Google Scholar]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef] [Green Version]
- Almeida, T.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Bacterial nanocellulose toward green cosmetics: Recent progresses and challenges. Int. J. Mol. Sci. 2021, 22, 2836. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Nanocellulose-based materials as components of polymer electrolyte fuel cells. J. Mater. Chem. A 2019, 7, 20045–20074. [Google Scholar] [CrossRef]
- Heise, K.; Kontturi, E.; Allahverdiyeva, Y.; Tammelin, T.; Linder, M.B.; Nonappa; Ikkala, O. Nanocellulose: Recent fundamental advances and emerging biological and biomimicking applications. Adv. Mater. 2020, 33, 2004349. [Google Scholar] [CrossRef]
- Meng, L.Y.; Wang, B.; Ma, M.G.; Zhu, J.F. Cellulose-based nanocarriers as platforms for cancer therapy. Curr. Pharm. Des. 2017, 23, 5292–5300. [Google Scholar] [CrossRef]
- Ul-islam, S.; Ul-islam, M.; Ahsan, H.; Ahmed, M.B.; Shehzad, A.; Fatima, A.; Sonn, J.K.; Lee, Y.S. Potential applications of bacterial cellulose and its composites for cancer treatment. Int. J. Biol. Macromol. 2021, 168, 301–309. [Google Scholar] [CrossRef]
- Seabra, A.B.; Bernardes, J.S.; Fávaro, W.J.; Paula, A.J.; Durán, N. Cellulose nanocrystals as carriers in medicine and their toxicities: A review. Carbohydr. Polym. 2018, 181, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.Q.; Vilela, C.; Santos, H.A.; Silvestre, A.J.D.; Freire, C.S.R. Recent trends on the development of systems for cancer diagnosis and treatment by microfluidic technology. Appl. Mater. Today 2020, 18, 100450. [Google Scholar] [CrossRef]
- World Health Organization Fact Sheets: Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 27 May 2021).
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today, Lyon, France: International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today (accessed on 27 May 2021).
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [Green Version]
- Gratton, S.E.A.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [Green Version]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, J.; Thomas, A.; Ou-Yang, D.; Muzykantov, V.R. The shape of things to come: Importance of design in nanotechnology for drug delivery. Ther. Deliv. 2012, 3, 181–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khine, Y.Y.; Stenzel, M.H. Surface modified cellulose nanomaterials: A source of non-spherical nanoparticles for drug delivery. Mater. Horiz. 2020, 7, 1727–1758. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.; Roman, M. Fluorescently labeled cellulose Nanocrystals for Bioimaging Applications. J. Am. Chem. Soc. 2007, 129, 13810–13811. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Dong, S.; Hirani, A.; Lee, Y.W. Cellulose nanocrystals for drug delivery. In Polysaccharide Materials: Performance by Design; Edgar, K.J., Heinze, T., Buchanan, C.M., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2010; Volume 1017, pp. 81–91. [Google Scholar]
- Dong, S.; Hirani, A.A.; Colacino, K.R.; Lee, Y.W.; Roman, M. Cytotoxicity and cellular uptake of cellulose nanocrystals. Nano Life 2012, 2, 1241006. [Google Scholar] [CrossRef]
- Dong, S.; Cho, H.J.; Lee, Y.W.; Roman, M. Synthesis and cellular uptake of folic acid-conjugated cellulose nanocrystals for cancer targeting. Biomacromolecules 2014, 15, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Colacino, K.R.; Arena, C.B.; Dong, S.; Roman, M.; Davalos, R.V.; Lee, Y.W. Folate conjugated cellulose nanocrystals potentiate irreversible electroporation-induced cytotoxicity for the selective treatment of cancer cells. Technol. Cancer Res. Treat. 2015, 14, 757–766. [Google Scholar] [CrossRef] [Green Version]
- Bittleman, K.R.; Dong, S.; Roman, M.; Lee, Y.W. Folic acid-conjugated cellulose nanocrystals show high folate-receptor binding affinity and uptake by KB and breast cancer cells. ACS Omega 2018, 3, 13952–13959. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.; Hamouda, A.E.I.; De Toledo, M.A.S.; Hu, C.; Bernardo, M.P.; Schalla, C.; Leite, L.S.F.; Buhl, E.M.; Dreschers, S.; Pich, A.; et al. Functionalized cellulose nanocrystals for cellular labeling and bioimaging. Biomacromolecules 2021, 22, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, S.; Buratti, V.V.; Maturi, M.; Sambri, L.; Franchini, M.C.; Locatelli, E. Surface-modified nanocellulose for application in biomedical engineering and nanomedicine: A review. Int. J. Nanomed. 2020, 15, 9909–9937. [Google Scholar] [CrossRef]
- Babu, A.; Ramesh, R. Multifaceted applications of chitosan in cancer drug delivery and therapy. Mar. Drugs 2017, 15, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wang, H.; Yu, G.; Yu, Q.; Li, B.; Mu, X. A novel approach for the preparation of nanocrystalline cellulose by using phosphotungstic acid. Carbohydr. Polym. 2014, 110, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lockey, R.; Mohapatra, S. Folate receptor-mediated cancer cell specific gene delivery using folic acid-conjugated oligochitosans. J. Nanosci. Nanotechnol. 2006, 6, 2860–2866. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Boamah, P.O.; Gong, J.; Zhang, Q.; Hua, M.; Ye, Y. Gd(III) complex conjugate of low-molecular-weight chitosan as a contrast agent for magnetic resonance/fluorescence dual-modal imaging. Carbohydr. Polym. 2016, 143, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Tobaldi, E.; Dovgan, I.; Mosser, M.; Becht, J.-M.; Wagner, A. Structural investigation of cyclo-dioxo maleimide cross-linkers for acid and serum stability. Org. Biomol. Chem. 2017, 15, 9305–9310. [Google Scholar] [CrossRef]
- Caprifico, A.E.; Polycarpou, E.; Foot, P.J.S.; Calabrese, G. Biomedical and pharmacological uses of fluorescein isothiocyanate chitosan-based nanocarriers. Macromol. Biosci. 2021, 21, 2000312. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Vilela, C.; Figueiredo, A.R.P.; Silvestre, A.J.D.; Freire, C.S.R. Multilayered materials based on biopolymers as drug delivery systems. Expert Opin. Drug Deliv. 2017, 14, 189–200. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, D.F.S.; Carvalho, J.P.F.; Bastos, V.; Oliveira, H.; Moreirinha, C.; Almeida, A.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Antibacterial multi-layered nanocellulose-based patches loaded with dexpanthenol for wound healing applications. Nanomaterials 2020, 10, 2469. [Google Scholar] [CrossRef]
- Chakraborty, P.; Bairi, P.; Roy, B.; Nandi, A.K. Improved mechanical and electronic properties of co-assembled folic acid gel with aniline and polyaniline. ACS Appl. Mater. Interfaces 2014, 6, 3615–3622. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Lee, T.; Han, H.; Go, W.J.; Yang, J.; Shin, H. Optical properties of fluorescein-labeled organoclay. Photochem. Photobiol. 2010, 86, 520–527. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Ali, M.; Chatterjee, H. Studies on the interaction of fluorescein isothiocyanate and its sugar analogues with cetyltrimethylammonium bromide. Chem. Phys. Lett. 2013, 561–562, 147–152. [Google Scholar] [CrossRef]
- Wang, L.; Roitberg, A.; Meuse, C.; Gaigalas, A.K. Raman and FTIR spectroscopies of fluorescein in solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 1781–1791. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules, 3rd ed.; Chapman and Hall, Ltd.: London, UK, 1975; ISBN 041-2-138-506. [Google Scholar]
- Alupei, L.; Lisa, G.; Butnariu, A.; Desbrières, J.; Cadinoiu, A.N.; Peptu, C.A.; Calin, G.; Popa, M. New folic acid-chitosan derivative based nanoparticles—Potential applications in cancer therapy. Cellul. Chem. Technol. 2017, 51, 631–648. [Google Scholar]
- Elshoky, H.A.; Salaheldin, T.A.; Ali, M.A.; Gaber, M.H. Ascorbic acid prevents cellular uptake and improves biocompatibility of chitosan nanoparticles. Int. J. Biol. Macromol. 2018, 115, 358–366. [Google Scholar] [CrossRef]
- Nawaz, A.; Wong, T.W. Chitosan-carboxymethyl-5-fluorouracil-folate conjugate particles: Microwave modulated uptake by skin and melanoma cells. J. Investig. Dermatol. 2018, 138, 2412–2422. [Google Scholar] [CrossRef] [Green Version]
- Filpponen, I.; Sadeghifar, H.; Argyropoulos, D.S. Photoresponsive cellulose nanocrystals. Nanomater. Nanotechnol. 2011, 1, 34–43. [Google Scholar] [CrossRef]
- Pinto, R.J.B.; Fernandes, S.C.M.; Freire, C.S.R.; Sadocco, P.; Causio, J.; Neto, C.P.; Trindade, T. Antibacterial activity of optically transparent nanocomposite films based on chitosan or its derivatives and silver nanoparticles. Carbohydr. Res. 2012, 348, 77–83. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, J. Preparation and characterization of the fluorescent chitosan nanoparticle probe. Chin. J. Anal. Chem. 2006, 34, 1555–1559. [Google Scholar] [CrossRef]
- Mahmoud, K.A.; Mena, J.A.; Male, K.B.; Hrapovic, S.; Kamen, A.; Luong, J.H.T. Effect of surface charge on the cellular uptake and cytotoxicity of fluorescent labeled cellulose nanocrystals. ACS Appl. Mater. Interfaces 2010, 2, 2924–2932. [Google Scholar] [CrossRef] [Green Version]
- Mohanta, V.; Madras, G.; Patil, S. Albumin-mediated incorporation of water-insoluble therapeutics in layer-by-layer assembled thin films and microcapsules. J. Mater. Chem. B 2013, 1, 4819–4827. [Google Scholar] [CrossRef]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Gorgieva, S.; Vivod, V.; Maver, U.; Gradišnik, L.; Dolenšek, J.; Kokol, V. Internalization of (bis)phosphonate-modified cellulose nanocrystals by human osteoblast cells. Cellulose 2017, 24, 4235–4252. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.L.; Moorefield, C.M.; Elbatal, H.S.; Newkome, G.R.; Modarelli, D.A.; Romano, N.C. Fluorescent cellulose nanocrystals via supramolecular assembly of terpyridine-modified cellulose nanocrystals and terpyridine-modified perylene. Mater. Sci. Eng. B 2012, 177, 350–358. [Google Scholar] [CrossRef]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2011, 32, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Meier, R.; Henning, T.D.; Boddington, S.; Tavri, S.; Arora, S.; Piontek, G.; Rudelius, M.; Corot, C.; Daldrup-Link, H.E. Breast Cancers: MR imaging of folate-receptor expression with the folate-specific nanoparticle P1133. Radiology 2010, 255, 527–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, V.; Kutty, R.V. Recent advances in nanotheranostics for triple negative breast cancer treatment. J. Exp. Clin. Cancer Res. 2019, 38, 430. [Google Scholar] [CrossRef] [Green Version]
- ISO 10993-5:2009(E). Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Hemraz, U.D.; Campbell, K.A.; Burdick, J.S.; Ckless, K.; Boluk, Y.; Sunasee, R. Cationic Poly(2-aminoethylmethacrylate) and Poly(N-(2-aminoethylmethacrylamide) modified cellulose nanocrystals: Synthesis, characterization, and cytotoxicity. Biomacromolecules 2015, 16, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Meschini, S.; Pellegrini, E.; Maestri, C.A.; Condello, M.; Bettotti, P.; Condello, G.; Scarpa, M. In vitro toxicity assessment of hydrogel patches obtained by cation-induced cross-linking of rod-like cellulose nanocrystals. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 687–697. [Google Scholar] [CrossRef]
- Reardon, A.J.F.; Elliott, J.A.W.; McGann, L.E. Fluorescence as an alternative to light-scatter gating strategies to identify frozen-thawed cells with flow cytometry. Cryobiology 2014, 69, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Guerra, Â.R.; Soares, B.I.G.; Freire, C.S.R.; Silvestre, A.J.D.; Duarte, M.F.; Duarte, I.F. Metabolic effects of a Eucalyptus bark lipophilic extract on triple negative breast cancer and nontumor breast epithelial cells. J. Proteome Res. 2021, 20, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibellini, F.; Smith, T.K. The Kennedy pathway—De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 2010, 62, 414–428. [Google Scholar] [CrossRef]
- Glunde, K.; Bhujwalla, Z.M.; Ronen, S.M. Choline metabolism in malignant transformation. Nat. Rev. Cancer 2011, 11, 835–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Cooper, D.E.; Cluntun, A.A.; Warmoes, M.O.; Zhao, S.; Reid, M.A.; Liu, J.; Lund, P.J.; Lopes, M.; Garcia, B.A.; et al. Acetate production from glucose and coupling to mitochondrial metabolism in mammals. Cell 2018, 175, 502–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamphorst, J.J.; Chung, M.K.; Fan, J.; Rabinowitz, J.D. Quantitative analysis of acetyl-CoA production in hypoxic cancer cells reveals substantial contribution from acetate. Cancer Metab. 2014, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Vines, J.B.; Yoon, J.H.; Ryu, N.E.; Lim, D.J.; Park, H. Gold nanoparticles for photothermal cancer therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Jaymand, M. Chemically modified natural polymer-based theranostic nanomedicines: Are they the golden gate toward a de novo clinical approach against cancer? ACS Biomater. Sci. Eng. 2020, 6, 134–166. [Google Scholar] [CrossRef]





| Nanosystems | Nominal Composition a | Measured Composition b | ||
|---|---|---|---|---|
| WCNCs (mg) | WFA-CS-FITC (mg) | WFA-CS-FITC (mg) | WFA-CS-FITC/WCNCs | |
| CNCs/FA-CS-FITC_1 | 50.0 | 1.01 | 0.65 | 0.013 |
| CNCs/FA-CS-FITC_2 | 50.0 | 1.99 | 1.43 | 0.029 |
| CNCs/FA-CS-FITC_3 | 50.0 | 4.09 | 2.97 | 0.059 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, R.J.B.; Lameirinhas, N.S.; Guedes, G.; Rodrigues da Silva, G.H.; Oskoei, P.; Spirk, S.; Oliveira, H.; Duarte, I.F.; Vilela, C.; Freire, C.S.R. Cellulose Nanocrystals/Chitosan-Based Nanosystems: Synthesis, Characterization, and Cellular Uptake on Breast Cancer Cells. Nanomaterials 2021, 11, 2057. https://doi.org/10.3390/nano11082057
Pinto RJB, Lameirinhas NS, Guedes G, Rodrigues da Silva GH, Oskoei P, Spirk S, Oliveira H, Duarte IF, Vilela C, Freire CSR. Cellulose Nanocrystals/Chitosan-Based Nanosystems: Synthesis, Characterization, and Cellular Uptake on Breast Cancer Cells. Nanomaterials. 2021; 11(8):2057. https://doi.org/10.3390/nano11082057
Chicago/Turabian StylePinto, Ricardo J. B., Nicole S. Lameirinhas, Gabriela Guedes, Gustavo H. Rodrigues da Silva, Párástu Oskoei, Stefan Spirk, Helena Oliveira, Iola F. Duarte, Carla Vilela, and Carmen S. R. Freire. 2021. "Cellulose Nanocrystals/Chitosan-Based Nanosystems: Synthesis, Characterization, and Cellular Uptake on Breast Cancer Cells" Nanomaterials 11, no. 8: 2057. https://doi.org/10.3390/nano11082057