Synthesis and Size Dependent Reflectance Study of Water Soluble SnS Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Properties





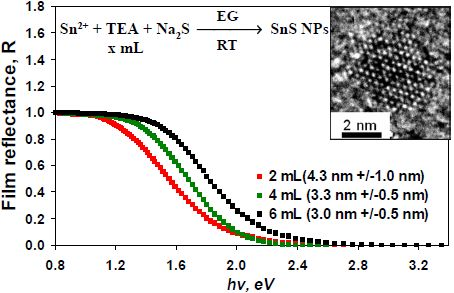
2.2. Effect of the Amount of TEA


2.3. Effect of Heat Treatment


3. Experimental Section
3.1. Materials
3.2. A Typical Synthesis of SnS Nanoparticles in the Presence of Triethanolamine
3.3. Heat Treatment
3.4. Characterization Methods
4. Conclusions
Acknowledgements
References
- Alivisatos, A.P. Semiconductor clusters, nanocrystals, and quantum dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kovalenko, M.V.; Shevchenko, E.V.; Talapin, D.V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]
- Leitsmann, R.; Bechstedt, F. Characteristic energies and shifts in optical spectra of colloidal IV−VI semiconductor nanocrystals. ACS Nano 2009, 3, 3505–3512. [Google Scholar] [CrossRef] [PubMed]
- Klimov, V.I.; Jeong, S.; Sykora, M.; Schaller, R.D. High-efficiency carrier multiplication and ultrafast charge separation in semiconductor nanocrystals studied via time-resolved photoluminescence. J. Phys. Chem. B 2006, 110, 25332–25338. [Google Scholar] [CrossRef] [PubMed]
- Nur, O.; Zhao, Q.X.; Asif, M.H.; Ali, S.U.; Wadeasa, A.; Yang, L.L.; Willander, M. Zinc oxide nanowires: Controlled low temperature growth and some electrochemical and optical nano-devices. J. Mater. Chem. 2009, 19, 1006–1018. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.Y.; Jiang, H.D.; Li, J.; Liu, D.; Cui, J.J.; Liu, X.Y.; Zhou, W.J. Control synthesis of rutile TiO2 microspheres, nanoflowers, nanotrees and nanobelts via acid-hydrothermal method and their optical properties. Cryst. Eng. Comm. 2011, 13, 4557–4563. [Google Scholar]
- Brutchey, R.L.; Buckley, J.J.; Antunez, P.D. Tin and germanium monochalcogenide IV–VI semiconductor nanocrystals for use in solar cells. Nanoscale 2011, 3, 2399–2411. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, A.P.; Aloni, S.; Amirav, L.; Jain, P.K. Nanoheterostructure cation exchange: Anionic framework conservation. J. Am. Chem. Soc. 2010, 132, 9997–9999. [Google Scholar] [CrossRef] [PubMed]
- Talapin, D.V.; Shevchenko, E.V.; Lee, J.-S. Au-PbS core-shell nanocrystals: Plasmonic absorption enhancement and electrical doping via intra-particle charge transfer. J. Am. Chem. Soc. 2008, 130, 9673–9675. [Google Scholar] [CrossRef] [PubMed]
- Brutchey, R.L.; Thompson, M.E.; Schlenker, C.W.; Franzman, M.A. Solution-phase synthesis of SnSe nanocrystals for use in solar cells. J. Am. Chem. Soc. 2010, 132, 4060–4061. [Google Scholar] [CrossRef] [PubMed]
- Hanrath, T.; Choi, J.J.; Baumgardner, W.J. SnSe nanocrystals: Synthesis, structure, optical properties, and surface chemistry. J. Am. Chem. Soc. 2010, 132, 9519–9521. [Google Scholar] [CrossRef] [PubMed]
- Odom, T.W.; Barton, J.E.; Greyson, E.C. Tetrahedral zinc blende tin sulfide nano- and microcrystals. Small 2006, 2, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Zainal, Z.; Hussein, M.Z.; Ghazali, A. Cathodic electrodeposition of SnS thin films from aqueous solution. Sol. Energy Mater. Sol. Cells 1996, 40, 347–357. [Google Scholar] [CrossRef]
- Carlone, C.; Parenteau, M. Influence of temperature and pressure on the electronic transitions in SnS and SnSe semiconductors. Phys. Rev. B 1990, 41, 5227–5234. [Google Scholar] [CrossRef]
- Ramakrishna Reddy, K.T.; Koteswara Reddy, N. Growth of polycrystalline SnS films by spray pyrolysis. Thin Solid Films 1998, 325, 4–6. [Google Scholar] [CrossRef]
- Poelman, D.; Tanusevski, A. Optical and photoconductive properties of SnS thin films prepared by electron beam evaporation. Sol. Energy Mater. Sol. Cells 2003, 80, 297–303. [Google Scholar] [CrossRef]
- Hu, G.X.; Fan, B.H.; Gong, H.; Wang, Y. Photovoltaic behavior of nanocrystalline SnS/TiO2. J. Phys. Chem. C 2010, 114, 3256–3259. [Google Scholar]
- Chaudhuri, S.; Gorai, S.; Panda, S.K. Shape selective solvothermal synthesis of SnS: Role of ethylenediamine-water solvent system. Mater. Sci. Eng. B 2006, 129, 265–269. [Google Scholar] [CrossRef]
- Qian, Y.; Zeng, J.; Yang, B.; Hu, H. Morphology evolution of SnS nanocrystals: From 3D urchin-like architectures to 1D nanostructures. Mater. Chem. Phys. 2004, 86, 233–237. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, D.; Zhu, H. 20. Zhu, H.; Yang, D.; Zhang, H.; Hydrothermal synthesis, characterization and properties of SnS nanoflowers. Mater. Lett. 2006, 60, 2686–2689. [Google Scholar] [CrossRef]
- Eychmüller, A.; Rellinghaus, B.; Waurisch, C.; Hickey, S.G. Size and shape control of colloidally synthesized IV−VI nanoparticulate tin(II) sulfide. J. Am. Chem. Soc. 2008, 130, 14978–14980. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Y.; Wang, Z.; He, P. Facile synthesis of monodisperse, size-tunable SnS nanoparticles potentially for solar cell energy conversion. Nanotechnology 2010, 21, 105707. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Liu, B.; Zou, B.; Dai, Q.; Wang, L.; Xiao, G.; Men, K.; Ning, J. Facile synthesis of IV–VI SnS nanocrystals with shape and size control: Nanoparticles, nanoflowers and amorphous nanosheets. Nanoscale 2010, 2, 1699–1703. [Google Scholar] [CrossRef] [PubMed]
- Rotello, V.M.; Ghosh, P.S.; De, M. Applications of nanoparticles in biology. Adv. Mater. 2008, 20, 4225–4241. [Google Scholar] [CrossRef]
- Tilley, R.D.; Bumby, C.W.; Al-Salim, N.; Xu, Y. Synthesis of SnS quantum dots. J. Am. Chem. Soc. 2009, 131, 15990–15991. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Ozin, G.A.; Verma, A.; Bedard, R.L. Adsorption and sensing properties of microporous layered tin sulfide materials. J. Mater. Chem. 1998, 8, 1649–1656. [Google Scholar] [CrossRef]
- P.; Basu, P.K.; Biswas, S. Preparation and characterization of chemically deposited tin(II) sulphide thin films. Thin Solid Films 1987, 150, 269–276. [Google Scholar] [CrossRef]
- Diffuse reflectance spectra were record on a High Accuracy Reference Spectrophotometer at the measurement standards laboratory of New Zealand (MSL)/Industrial Research Limited.
- Yang, W.; Gao, F.; Wei, G.; An, L. Ostwald ripening growth of silicon nitride nanoplates. Cryst. Growth Des. 2010, 10, 29–31. [Google Scholar] [CrossRef]
- Chergui, M.; Pattison, P.; Al-Salman, A.; Mohammed, M.B.; Tonti, D. Multimodal distribution of quantum confinement in ripened CdSe nanocrystals. Chem. Mater. 2008, 20, 1331–1339. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Zhong, X. Facile and reproducible synthesis of red-emitting CdSe nanocrystals in amine with long-term fixation of particle size and size distribution. J. Phys. Chem. C 2007, 111, 526–531. [Google Scholar]
- Vlachos, D.G.; Lobo, R.F.; Navrotsky, A.; Trofymluk, O.; Rimer, J.D. Kinetic and thermodynamic studies of silica nanoparticle dissolution. Chem. Mater. 2007, 19, 4189–4197. [Google Scholar] [CrossRef]
- Tilley, R.D.; Hodgkiss, J.M.; Al-Salim, N.; Xu, Y. Solution synthesis and optical properties of SnTe nanocrystals. Cryst. Growth Des. 2011, 11, 2721–2723. [Google Scholar] [CrossRef]
- Tong, Y.; Xu, M.; Wang, K.; Pan, G.; Cao, F.; Chen, H.; Tang, P. Nanoparticulate SnS as an efficient photocatalyst under visible-light irradiation. Mater. Lett. 2011, 65, 450–452. [Google Scholar] [CrossRef]
- Shen, Z.; Sun, L.; Shen, H.; Gao, C. Chemical bath deposition of SnS films with different crystal structures. Mater. Lett. 2011, 65, 1413–1415. [Google Scholar] [CrossRef]
- Mohandas, E.; Arora, A.K.; Muralidharan, N.G.; Venkiteswaran, C.N.; Divakar, R.; Ghosh, C.; Kalavathi, S.; Muthamizhchelvan, C.; Rajalakshmi, M.; Sohila, S. Synthesis and characterization of SnS nanosheets through simple chemical route. Mater. Lett. 2011, 65, 1148–1150. [Google Scholar] [CrossRef]
- Gunasekhar, K.R.; Ahsanulhaq, Q.; Devika, M.; Koteeswara Reddy, N. Growth of orthorhombic SnS nanobox structures on seeded substrates. Cryst. Growth Des. 2010, 10, 4769–4772. [Google Scholar] [CrossRef]
- Tatsumisago, M.; Hayashi, A.; Aso, K. Synthesis of needlelike and platelike SnS active materials in high-boiling solvents and their application to all-solid-state lithium secondary batteries. Cryst. Growth Des. 2011, 11, 3900–3904. [Google Scholar] [CrossRef]
© 2012 by the authors. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, Y.; Al-Salim, N.; Tilley, R.D. Synthesis and Size Dependent Reflectance Study of Water Soluble SnS Nanoparticles. Nanomaterials 2012, 2, 54-64. https://doi.org/10.3390/nano2010054
Xu Y, Al-Salim N, Tilley RD. Synthesis and Size Dependent Reflectance Study of Water Soluble SnS Nanoparticles. Nanomaterials. 2012; 2(1):54-64. https://doi.org/10.3390/nano2010054
Chicago/Turabian StyleXu, Ying, Najeh Al-Salim, and Richard D. Tilley. 2012. "Synthesis and Size Dependent Reflectance Study of Water Soluble SnS Nanoparticles" Nanomaterials 2, no. 1: 54-64. https://doi.org/10.3390/nano2010054