Surface Modification of Magnetic Iron Oxide Nanoparticles
Abstract
:1. Introduction
2. Surface Modification of Magnetic Iron Oxide Nanoparticles (IONPs) and Applications
2.1. Surface Coating with Inorganic Materials
2.1.1. Silica
2.1.2. Carbon
2.1.3. Metal
2.1.4. Metal Oxides/Sulfides
2.2. Surface Coating with Organic Materials
2.2.1. Polymers
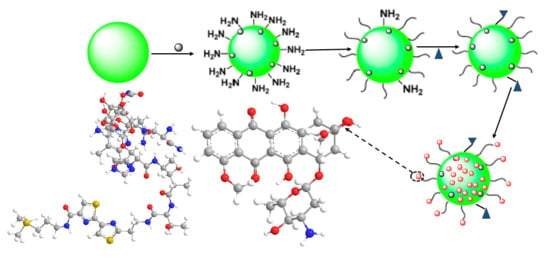
2.2.2. Small Molecules and Surfactants
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rossi, L.M.; Costa, N.J.S.; Silva, F.P.; Wojcieszak, R. Magnetic nanomaterials in catalysis: Advanced catalysts for magnetic separation and beyond. Green Chem. 2014, 16, 2906–2933. [Google Scholar] [CrossRef]
- Zhang, H.W.; Liu, Y.; Sun, S.H. Synthesis and assembly of magnetic nanoparticles for information and energy storage applications. Front. Phys. Chin. 2010, 5, 347–356. [Google Scholar] [CrossRef]
- Tang, S.C.N.; Lo, I.M.C. Magnetic nanoparticles: Essential factors for sustainable environmental applications. Water Res. 2013, 47, 2613–2632. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Dutz, S.; Hafeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface 2011, 166, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-B.; Gao, Y.-J.; Wang, Y.; Zhan, F.; Zhang, X.-Y.; Kong, Q.-Y.; Zhao, N.-J.; Guo, Q.; Wu, H.-L.; Li, Z.-J.; et al. Self-Assembled Framework Enhances Electronic Communication of Ultrasmall-Sized Nanoparticles for Exceptional Solar Hydrogen Evolution. J. Am. Chem. Soc. 2017, 139, 4789–4796. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Gao, J.H.; Ai, H.; Chen, X.Y. Applications and Potential Toxicity of Magnetic Iron Oxide Nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H.; Park, M.; Do, M.J.; Lee, N.; Ryu, J.H.; Kim, G.W.; Kim, C.; Park, T.G.; Hyeon, T. Chitosan Oligosaccharide-Stabilized Ferrimagnetic Iron Oxide Nanocubes for Magnetically Modulated Cancer Hyperthermia. ACS Nano 2012, 6, 5266–5273. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine 2016, 11, 1889–1910. [Google Scholar] [CrossRef] [PubMed]
- Gruttner, C.; Muller, K.; Teller, J.; Westphal, F. Synthesis and functionalisation of magnetic nanoparticles for hyperthermia applications. Int. J. Hyperther. 2013, 29, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Escribano, E.; Queralt, J.; Antonia Busquets, M. Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Shen, S.Z.; Sun, H.; Sun, K.; Liu, F.; Qi, Y.; Yan, J. Design and construction of polymerized-chitosan coated Fe3O4 magnetic nanoparticles and its application for hydrophobic drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Wahajuddin, S.A. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Hyeon, T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2012, 41, 2575–2589. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, N.; Kim, H.; An, K.; Park, Y.I.; Choi, Y.; Shin, K.; Lee, Y.; Kwon, S.G.; Na, H.B.; et al. Large-Scale Synthesis of Uniform and Extremely Small-Sized Iron Oxide Nanoparticles for High-Resolution T-1 Magnetic Resonance Imaging Contrast Agents. J. Am. Chem. Soc. 2011, 133, 12624–12631. [Google Scholar] [CrossRef] [PubMed]
- Fatima, H.; Kim, K.S. Magnetic nanoparticles for bioseparation. Korean J. Chem. Eng. 2017, 34, 589–599. [Google Scholar] [CrossRef]
- Kannan, K.; Mukherjee, J.; Gupta, M.N. Use of Polyethyleneimine Coated Fe3O4 Nanoparticles as an Ion-Exchanger for Protein Separation. Sci. Adv. Mater. 2013, 5, 1477–1484. [Google Scholar] [CrossRef]
- Zhang, G.X.; Qie, F.X.; Hou, J.X.; Luo, S.Z.; Luo, L.; Sun, X.M.; Tan, T.W. One-pot solvothermal method to prepare functionalized Fe3O4 nanoparticles for bioseparation. J. Mater. Res. 2012, 2, 1006–1013. [Google Scholar] [CrossRef]
- Jiang, S.; Eltoukhy, A.A.; Love, K.T.; Langer, R.; Anderson, D.G. Lipidoid-Coated Iron Oxide Nanoparticles for Efficient DNA and siRNA delivery. Nano Lett. 2012, 13, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Kami, D.; Takeda, S.; Itakura, Y.; Gojo, S.; Watanabe, M.; Toyoda, M. Application of Magnetic Nanoparticles to Gene Delivery. Int. J. Mol. Sci. 2011, 12, 3705–3722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mykhaylyk, O.; Sobisch, T.; Almstatter, I.; Sanchez-Antequera, Y.; Brandt, S.; Anton, M.; Doblinger, M.; Eberbeck, D.; Settles, M.; Braren, R.; et al. Silica-Iron Oxide Magnetic Nanoparticles Modified for Gene Delivery: A Search for Optimum and Quantitative Criteria. Pharm. Res. 2012, 29, 1344–1365. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Salazar, P.; Villalonga, R.; Campuzano, S.; Pingarron, J.M.; Gonzalez-Mora, J.L. Preparation of core-shell Fe3O4@poly(dopamine) magnetic nanoparticles for biosensor construction. J. Mater. Chem. B 2014, 2, 739–746. [Google Scholar] [CrossRef]
- Shi, X.H.; Gu, W.; Li, B.Y.; Chen, N.N.; Zhao, K.; Xian, Y.Z. Enzymatic biosensors based on the use of metal oxide nanoparticles. Microchim. Acta 2014, 181, 1–22. [Google Scholar] [CrossRef]
- Baghayeri, M.; Zare, E.N.; Lakouraj, M.M. A simple hydrogen peroxide biosensor based on a novel electro-magnetic poly(p-phenylenediamine)@Fe3O4 nanocomposite. Biosens. Bioelectron. 2014, 55, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Li, Z.H.; Wang, J.L.; Ge, W.P.; Yue, T.L.; Li, R.H.; Colvin, V.L.; Yu, W.W. Food related applications of magnetic iron oxide nanoparticles: Enzyme immobilization, protein purification, and food analysis. Trends Food Sci. Tech. 2012, 27, 47–56. [Google Scholar] [CrossRef]
- Okoli, C.; Boutonnet, M.; Mariey, L.; Jaras, S.; Rajarao, G. Application of magnetic iron oxide nanoparticles prepared from microemulsions for protein purification. J. Chem. Technol. Biotechnol. 2011, 86, 1386–1393. [Google Scholar] [CrossRef]
- Okoli, C.; Fornara, A.; Qin, J.; Toprak, M.S.; Dalhammar, G.; Muhammed, M.; Rajarao, G.K. Characterization of Superparamagnetic Iron Oxide Nanoparticles and Its Application in Protein Purification. J. Nanosci. Nanotechnol. 2011, 11, 10201–10206. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.D.; Chen, W.L.; Cunningham, B.T.; Andrade, J.E. Enhanced sandwich immunoassay using antibody-functionalized magnetic iron-oxide nanoparticles for extraction and detection of soluble transferrin receptor on a photonic crystal biosensor. Biosens. Bioelectron. 2015, 74, 815–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nor, N.M.; Razak, K.A.; Tan, S.C.; Noordin, R. Properties of surface functionalized iron oxide nanoparticles (ferrofluid) conjugated antibody for lateral flow immunoassay application. J. Alloys Compd. 2012, 538, 100–106. [Google Scholar]
- Yang, M.Z.; Guan, Y.P.; Yang, Y.; Xia, T.T.; Xiong, W.B.; Guo, C. A sensitive and rapid immunoassay for mycoplasma pneumonia based on Fe3O4 nanoparticles. Mater. Lett. 2014, 137, 113–116. [Google Scholar] [CrossRef]
- Li, L.; Jiang, W.; Luo, K.; Song, H.; Lan, F.; Wu, Y.; Gu, Z. Superparamagnetic Iron Oxide Nanoparticles as MRI contrast agents for Non-invasive Stem Cell Labeling and Tracking. Theranostics 2013, 3, 595–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.Q.; Wei, F.; Liu, A.J.; Wang, L.; Wang, J.C.; Ren, L.; Liu, W.M.; Tu, Q.; Li, L.; Wang, J.Y. Cancer stem cell labeling using poly(L-lysine)-modified iron oxide nanoparticles. Biomaterials 2012, 33, 3719–3732. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.H.; Himmelreich, U.; Nuytten, N.; De Cuyper, M. Cytotoxic effects of iron oxide nanoparticles and implications for safety in cell labelling. Biomaterials 2011, 32, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Barikani, M.; Barmar, M. Effect of surface modification of Fe3O4 nanoparticles on thermal and mechanical properties of magnetic polyurethane elastomer nanocomposites. J. Mater. Sci. 2013, 48, 7493–7502. [Google Scholar] [CrossRef]
- Sun, S.N.; Wei, C.; Zhu, Z.Z.; Hou, Y.L.; Venkatraman, S.S.; Xu, Z.C. Magnetic iron oxide nanoparticles: Synthesis and surface coating techniques for biomedical applications. Chin. Phys. B 2014, 23. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B-Adv. 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Li, H.; Lu, Z.; Cheng, G.; Rong, K.F.; Chen, F.X.; Chen, R. HEPES-involved hydrothermal synthesis of Fe3O4 nanoparticles and their biological application. RSC Adv. 2015, 5, 5059–5067. [Google Scholar] [CrossRef]
- Sharma, G.; Jeevanandam, P. Synthesis of self-assembled prismatic iron oxide nanoparticles by a novel thermal decomposition route. RSC Adv. 2013, 3, 189–200. [Google Scholar] [CrossRef]
- Okoli, C.; Sanchez-Dominguez, M.; Boutonnet, M.; Jaras, S.; Civera, C.; Solans, C.; Kuttuva, G.R. Comparison and Functionalization Study of Microemulsion-Prepared Magnetic Iron Oxide Nanoparticles. Langmuir 2012, 28, 8479–8485. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, I.; Aghazadeh, M.; Doroudi, T.; Ganjali, M.R.; Kolivand, P.H. Effective Preparation, Characterization and In Situ Surface Coating of Superparamagnetic Fe3O4 Nanoparticles with Polyethyleneimine Through Cathodic Electrochemical Deposition (CED). Curr. Nanosci. 2017, 13, 167–174. [Google Scholar] [CrossRef]
- Morjan, I.; Alexandrescu, R.; Dumitrache, F.; Birjega, R.; Fleaca, C.; Soare, I.; Luculescu, C.R.; Filoti, G.; Kuncer, V.; Vekas, L.; et al. Iron Oxide-Based Nanoparticles with Different Mean Sizes Obtained by the Laser Pyrolysis: Structural and Magnetic Properties. J. Nanosci. Nanotechnol. 2010, 10, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Ooi, F.; DuChene, J.S.; Qiu, J.J.; Graham, J.O.; Engelhard, M.H.; Cao, G.X.; Gai, Z.; Wei, W.D. A Facile Solvothermal Synthesis of Octahedral Fe3O4 Nanoparticles. Small 2015, 11, 2649–2653. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Takahashi, M.; Kim, C. Facile sonochemical synthesis of high-moment magnetite (Fe3O4) nanocube. J. Nanopart. Res. 2013, 15, 1354. [Google Scholar] [CrossRef]
- Wang, J.H.; Gao, M.X.; Wang, D.S.; Li, X.; Dou, Y.B.; Liu, Y.F.; Pan, H.G. Chemical vapor deposition prepared bi-morphological carbon-coated Fe3O4 composites as anode materials for lithium-ion batteries. J. Power Sources 2015, 282, 257–264. [Google Scholar] [CrossRef]
- Solano, E.; Perez-Mirabet, L.; Martinez-Julian, F.; Guzman, R.; Arbiol, J.; Puig, T.; Obradors, X.; Yanez, R.; Pomar, A.; Ricart, S.; et al. Facile and efficient one-pot solvothermal and microwave-assisted synthesis of stable colloidal solutions of MFe2O4 spinel magnetic nanoparticles. J. Nanopart. Res. 2012, 14, 15. [Google Scholar] [CrossRef]
- Strobel, R.; Pratsinis, S.E. Flame aerosol synthesis of smart nanostructured materials. J. Mater. Chem. 2007, 17, 4743–4756. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A. Fe3O4/SiO2 nanoparticles: An efficient and magnetically recoverable nanocatalyst for the one-pot multicomponent synthesis of diazepines. Tetrahedron 2012, 68, 7827–7833. [Google Scholar] [CrossRef]
- Kiasat, A.R.; Davarpanah, J. Fe3O4@silica sulfuric acid nanoparticles: An efficient reusable nanomagnetic catalyst as potent solid acid for one-pot solvent-free synthesis of indazolo 2,1-b phthalazine-triones and pyrazolo 1,2-b phthalazine-diones. J. Mol. Catal. A-Chem. 2013, 373, 46–54. [Google Scholar] [CrossRef]
- Sun, H.; Zeng, X.; Liu, M.; Elingarami, S.; Li, G.; Shen, B.; He, N. Synthesis of Size-Controlled Fe3O4@SiO2 Magnetic Nanoparticles for Nucleic Acid Analysis. J. Nanosci. Nanotechnol. 2012, 12, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.X.; Sun, W.Z.; Kessler, M.R.; Bowler, N.; Dennis, K.W.; McCallum, R.W.; Li, Q.; Tan, X.L. Multifunctional Properties of Cyanate Ester Composites with SiO2 Coated Fe3O4 Fillers. ACS Appl. Mater. Int. 2013, 5, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Ma, C.; Wang, F.; Xi, Z.J.; Wang, Z.F.; Deng, Y.; He, N.Y. Preparation and Biomedical Applications of Core-Shell Silica/Magnetic Nanoparticle Composites. J. Nanosci. Nanotechnol. 2012, 12, 2964–2972. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Rao, B.P.; Islam, M.N.; Naga, S.M.; Takahashi, M.; Kim, C. Highly stable-silica encapsulating magnetite nanoparticles (Fe3O4/SiO2) synthesized using single surfactantless-polyol process. Ceram. Int. 2014, 40, 1379–1385. [Google Scholar] [CrossRef]
- Chu, X.; Yu, J.; Hou, Y.-L. Surface modification of magnetic nanoparticles in biomedicine. Chinese. Phys. B. 2015, 24, 014704. [Google Scholar] [CrossRef]
- Hui, C.; Shen, C.M.; Tian, J.F.; Bao, L.H.; Ding, H.; Li, C.; Tian, Y.A.; Shi, X.Z.; Gao, H.J. Core-shell Fe3O4@SiO2 nanoparticles synthesized with well-dispersed hydrophilic Fe3O4 seeds. Nanoscale 2011, 3, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yu, J.G.; Chang, B.; Zhao, X.J. Preparation and formation mechanism of monodispersed silicon dioxide spherical particles. Acta Chim. Sin. 2003, 61, 562–566. [Google Scholar]
- Malvindi, M.A.; De Matteis, V.; Galeone, A.; Brunetti, V.; Anyfantis, G.C.; Athanassiou, A.; Cingolani, R.; Pompa, P.P. Toxicity assessment of silica coated iron oxide nanoparticles and biocompatibility improvement by surface engineering. PLoS ONE 2014, 9, e85835. [Google Scholar] [CrossRef] [PubMed]
- Uribe Madrid, S.I.; Pal, U.; Kang, Y.S.; Kim, J.; Kwon, H.; Kim, J. Fabrication of Fe3O4@mSiO2 Core-Shell Composite Nanoparticles for Drug Delivery Applications. Nanoscale Res. Lett. 2015, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, M.; Georgescu, M.; Alexandrescu, L.; Gurau, D.; Ficai, A.; Ficai, D.; Andronescu, E. Synthesis and applications of Fe3O4/SiO2 core-shell materials. Curr. Pharm. Des. 2015, 21, 5324–5335. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, L.; Leung, C.W.; Lai, P.T.; Pong, P.W.T. Synthesis and Characterization of Silica-Encapsulated Iron Oxide Nanoparticles. IEEE Trans. Magn. 2014, 50, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Shen, D.; Wei, Y.; Li, W.; Zhang, F.; Kong, B.; Zhang, S.; Teng, W.; Fan, J.; Zhang, W.; et al. Monodisperse core-shell structured magnetic mesoporous aluminosilicate nanospheres with large dendritic mesochannels. Nano Res. 2015, 8, 2503–2514. [Google Scholar] [CrossRef]
- Ding, H.L.; Zhang, Y.X.; Wang, S.; Xu, J.M.; Xu, S.C.; Li, G.H. Fe3O4@SiO2 Core/Shell Nanoparticles: The Silica Coating Regulations with a Single Core for Different Core Sizes and Shell Thicknesses. Chem. Mater. 2012, 24, 4572–4580. [Google Scholar] [CrossRef]
- Lu, C.Y.; Puig, T.; Obradors, X.; Ricart, S.; Ros, J. Ultra-fast microwave-assisted reverse microemulsion synthesis of Fe3O4@SiO2 core-shell nanoparticles as a highly recyclable silver nanoparticle catalytic platform in the reduction of 4-nitroaniline. RSC Adv. 2016, 6, 88762–88769. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Jiang, H.; Li, C. Double-faced γ-Fe2O3||SiO2 nanohybrids: Flame synthesis, in situ selective modification and highly interfacial activity. Nanoscale 2013, 5, 5360. [Google Scholar] [CrossRef] [PubMed]
- Tricoli, A.; Righettoni, M.; Krumeich, F.; Stark, W.J.; Pratsinis, S.E. Scalable flame synthesis of SiO2 nanowires: Dynamics of growth. Nanotechnology 2010, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Tani, T.; Madler, L.; Pratsinis, S.E. Homogeneous ZnO nanoparticles by flame spray pyrolysis. J. Nanopart. Res. 2002, 4, 337–343. [Google Scholar] [CrossRef]
- Jokanovic, V.; Spasic, A.M.; Uskokovic, D. Designing of nanostructured hollow TiO2 spheres obtained by ultrasonic spray pyrolysis. J. Colloid Interfaces Sci. 2004, 278, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Strobel, R.; Pratsinis, S.E. Direct synthesis of maghemite, magnetite and wustite nanoparticles by flame spray pyrolysis. Adv. Powder Technol. 2009, 20, 190–194. [Google Scholar] [CrossRef]
- Kelesidis, G.A.; Furrer, F.M.; Wegner, K.; Pratsinis, S.E. Impact of Humidity on Silica Nanoparticle Agglomerate Morphology and Size Distribution. Langmuir 2018, 34, 8532–8541. [Google Scholar] [CrossRef] [PubMed]
- Teleki, A.; Suter, M.; Kidambi, P.; Ergeneman, O.; Krumeich, F.; Nelson, B.; Pratsinis, S. Hermetically Coated Superparamagnetic Fe2O3 Particles with SiO2 Nanofilms. Chem. Mater. 2009, 21, 2094–2100. [Google Scholar] [CrossRef]
- Setyawan, H.; Fajaroh, F.; Widiyastuti, W.; Winardi, S.; Lenggoro, I.W.; Mufti, N. One-step synthesis of silica-coated magnetite nanoparticles by electrooxidation of iron in sodium silicate solution. J. Nanopart. Res. 2012, 14, 807. [Google Scholar] [CrossRef]
- Roto, R.; Yusran, Y.; Kuncaka, A. Magnetic adsorbent of Fe3O4@SiO2 core-shell nanoparticles modified with thiol group for chloroauric ion adsorption. Appl. Surf. Sci. 2016, 377, 30–36. [Google Scholar] [CrossRef]
- Fajaroh, F.; Setyawan, H.; Widiyastuti, W.; Winardi, S. Synthesis of magnetite nanoparticles by surfactant-free electrochemical method in an aqueous system. Adv. Powder Technol. 2012, 23, 328–333. [Google Scholar] [CrossRef]
- Fajaroh, F.; Sumari, N. Effect of concentration of sodium silicate solution in the synthesis of silica-coated magnetite nanoparticles by ultrasonication. In Proceedings of the 6th Nanoscience and Nanotechnology Symposium, Surakarta, Indonesia, 4–5 November 2015; American Institute of Physics: Melville, NY, USA, 2016. [Google Scholar]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2010, 110, 2574. [Google Scholar] [CrossRef]
- Yang, H.; Zhuang, Y.M.; Sun, Y.; Dai, A.T.; Shi, X.Y.; Wu, D.M.; Li, F.Y.; Hu, H.; Yang, S.P. Targeted dual-contrast T-1- and T-2-weighted magnetic resonance imaging of tumors using multifunctional gadolinium-labeled superparamagnetic iron oxide nanoparticles. Biomaterials 2011, 32, 4584–4593. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Pauletti, G.M.; Wang, J.T.; Zhang, J.M.; Ewing, R.C.; Wang, Y.L.; Shi, D.L. Dual Surface-Functionalized Janus Nanocomposites of Polystyrene/Fe3O4@SiO2 for Simultaneous Tumor Cell Targeting and Stimulus-Induced Drug Release. Adv. Mater. 2013, 25, 3485–3489. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Zheng, S.R.; Shao, Y.; Liu, J.L.; Xu, Z.Y.; Zhu, D.Q. Amino-functionalized Fe3O4@SiO2 core-shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J. Colloid Interfaces Sci. 2010, 349, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; He, M.L.; Liu, J.H.; Liao, R.; Zhao, L.Q.; Xie, J.R.; Wang, R.J.; Yang, S.T.; Wang, H.F.; Liu, Y.F. Fe3O4@C nanoparticles as high-performance Fenton-like catalyst for dye decoloration. Chin. Sci. Bull. 2014, 59, 3406–3412. [Google Scholar] [CrossRef]
- Lim, Y.S.; Lai, C.W.; Hamid, S.B.A. Porous 3D carbon decorated Fe3O4 nanocomposite electrode for highly symmetrical supercapacitor performance. RSC Adv. 2017, 7, 23030–23040. [Google Scholar] [CrossRef]
- Wang, J.C.; Zhou, H.; Zhuang, J.D.; Liu, Q. Magnetic gamma-Fe2O3, Fe3O4, and Fe nanoparticles confined within ordered mesoporous carbons as efficient microwave absorbers. Phys. Chem. Chem. Phys. 2015, 17, 3802–3812. [Google Scholar] [CrossRef] [PubMed]
- He, C.N.; Wu, S.; Zhao, N.Q.; Shi, C.S.; Liu, E.Z.; Li, J.J. Carbon-Encapsulated Fe3O4 Nanoparticles as a High-Rate Lithium Ion Battery Anode Material. ACS Nano. 2013, 7, 4459–4469. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.C.; Liu, W.W.; Qiang, R.; Wang, Y.; Han, X.J.; Ma, J.; Xu, P. Shell Thickness-Dependent Microwave Absorption of Core-Shell Fe3O4@C Composites. ACS Appl. Mater. Int. 2014, 6, 12997–13006. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, Y.C.; Liu, F.; Liu, C.P.; Wang, J.B.; Pan, Y.; Xue, D.F. One-pot synthesis of mesoporous interconnected carbon-encapsulated Fe3O4 nanospheres as superior anodes for Li-ion batteries. RSC Adv. 2012, 2, 2262–2265. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.Q.; Zhuang, S.X.; Wang, X.W.; Tu, F.Y. Synthesis of carbon-coated Fe3O4 nanorods as electrode material for supercapacitor. Ionics 2013, 19, 1255–1261. [Google Scholar] [CrossRef]
- Sinan, N.; Unur, E. Fe3O4/carbon nanocomposite: Investigation of capacitive & magnetic properties for supercapacitor applications. Mater. Chem. Phys. 2016, 183, 571–579. [Google Scholar]
- Wang, Y.H.; He, P.; Zhao, X.M.; Lei, W.; Dong, F.Q. Coal tar residues-based nanostructured activated carbon/Fe3O4 composite electrode materials for supercapacitors. J. Solid State Electr. 2014, 18, 665–672. [Google Scholar] [CrossRef]
- Guo, C.; Wang, L.L.; Zhu, Y.C.; Wang, D.F.; Yang, Q.Q.; Qian, Y.T. Fe3O4 nanoflakes in an N-doped carbon matrix as high-performance anodes for lithium ion batteries. Nanoscale 2015, 7, 10123–10129. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Xiao, C.H.; Chen, X.; Gao, R.X.; Ding, S.J. Porous gamma-Fe2O3 spheres coated with N-doped carbon from polydopamine as Li-ion battery anode materials. Nanotechnology 2016, 27, 215403. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.T.; Zhu, W.K.; Liu, W.L.; Kong, F.G.; Ren, M.M.; Liu, Q.Z.; Yang, Z.Z.; Wang, X.Q.; Duan, X.L. Preparation of yolk-shell Fe3O4@N-doped carbon nanocomposite particles as anode in lithium ion batteries. J. Mater. Sci. 2017, 28, 11569–11575. [Google Scholar] [CrossRef]
- Yang, L.; Guo, G.N.; Sun, H.J.; Shen, X.D.; Hu, J.H.; Dong, A.G.; Yang, D. Ionic Liquid as the C and N Sources to Prepare Yolk-shell Fe3O4@N-doped Carbon Nanoparticles and its High Performance in Lithium-ion Battery. Electrochim. Acta 2016, 190, 797–803. [Google Scholar] [CrossRef]
- Hadidi, L.; Davari, E.; Ivey, D.G.; Veinot, J.G.C. Microwave-assisted synthesis and prototype oxygen reduction electrocatalyst application of N-doped carbon-coated Fe3O4 nanorods. Nanotechnology 2017, 28, 095707. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Si, J.J.; Li, J.; Wang, Q.; Chen, S.L. Hybrid of Fe3O4 nanorods and N-doped carbon as efficient oxygen reduction electrocatalyst. Int. J. Hydrog. Energy 2016, 41, 16858–16864. [Google Scholar] [CrossRef]
- Guan, D.H.; Gao, Z.; Yang, W.L.; Wang, J.; Yuan, Y.; Wang, B.; Zhang, M.L.; Liu, L.H. Hydrothermal synthesis of carbon nanotube/cubic Fe3O4 nanocomposite for enhanced performance supercapacitor electrode material. Mat. Sci. Eng. B 2013, 178, 736–743. [Google Scholar] [CrossRef]
- Chen, M.L.; He, Y.J.; Chen, X.W.; Wang, J.H. Quantum Dots Conjugated with Fe3O4-Filled Carbon Nanotubes for Cancer-Targeted Imaging and Magnetically Guided Drug Delivery. Langmuir 2012, 28, 16469–16476. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wei, Y.; Wang, J.P.; Jiang, K.L.; Fan, S.S. Conformal Fe3O4 Sheath on Aligned Carbon Nanotube Scaffolds as High-Performance Anodes for Lithium Ion Batteries. Nano Lett. 2013, 13, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Hu, L.; Yang, F.; Guo, T. Improvement of the field emission properties of carbon nanotubes by CNT/Fe3O4composite electrophoretic deposition. J. Semicond. 2011, 32, 126001. [Google Scholar] [CrossRef]
- Guo, Q.X.; Guo, P.F.; Li, J.T.; Yin, H.; Liu, J.; Xiao, F.L.; Shen, D.X.; Li, N. Fe3O4-CNTs nanocomposites: Inorganic dispersant assisted, hydrothermal synthesis and application in lithium ion batteries. J. Solid State Chem. 2014, 213, 104–109. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Zeng, X.J.; Chen, M.; Yu, R.H. Controllable permittivity in 3D Fe3O4/CNTs network for remarkable microwave absorption performances. RSC Adv. 2017, 7, 26801–26808. [Google Scholar] [CrossRef]
- Zhang, X.J.; Hao, L.Y.; Wang, H.H.; Zhu, X.Q.; Zhang, Z.Y.; Hu, X.H.; Jiang, W. Preparation and Characterization of Superparamagnetic Fe3O4/CNTs Nanocomposites Dual-drug Carrier. J. Wuhan Univ. Technol. 2017, 32, 42–46. [Google Scholar] [CrossRef]
- Alegret, N.; Criado, A.; Prato, M. Recent Advances of Graphene-based Hybrids with Magnetic Nanoparticles for Biomedical Applications. Curr. Med. Chem. 2017, 24, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yang, S.B.; Zhou, H.X.; Lieberwirth, I.; Feng, X.L.; Mullen, K. 3D Graphene Foams Cross-linked with Pre-encapsulated Fe3O4 Nanospheres for Enhanced Lithium Storage. Adv. Mater. 2013, 25, 2909–2914. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.S.; Xue, X.Q.; Jiang, G.M.; Wu, D.L.; Sheng, T.T.; Zhou, H.Y.; Xu, X.H. Nanoscale Zero-Valent Iron (nZVI) assembled on magnetic Fe3O4/graphene for Chromium (VI) removal from aqueous solution. J. Colloid Interfaces Sci. 2014, 417, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.Q.; Xi, P.X.; Liu, H.Y.; Chen, F.J.; Huang, L.; Shi, Y.J.; Hou, F.P.; Zeng, Z.Z.; Shao, C.W.; Wang, J. A facile chemical method to produce superparamagnetic graphene oxide-Fe3O4 hybrid composite and its application in the removal of dyes from aqueous solution. J. Mater. Chem. 2012, 22, 1033–1039. [Google Scholar] [CrossRef]
- Zubir, N.A.; Yacou, C.; Motuzas, J.; Zhang, X.W.; Zhao, X.S.; da Costa, J.C.D. The sacrificial role of graphene oxide in stabilising a Fenton-like catalyst GO-Fe3O4. Chem. Commun. 2015, 51, 9291–9293. [Google Scholar] [CrossRef] [PubMed]
- Teymourian, H.; Salimi, A.; Khezrian, S. Fe3O4 magnetic nanoparticles/reduced graphene oxide nanosheets as a novel electrochemical and bioeletrochemical sensing platform. Biosens. Bioelectron. 2013, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.; Kalpana, D.; Amaresh, S.; Lee, Y.S. Microwave synthesis of graphene/magnetite composite electrode material for symmetric supercapacitor with superior rate performance. RSC Adv. 2012, 2, 12322–12328. [Google Scholar] [CrossRef]
- Hu, C.G.; Mou, Z.Y.; Lu, G.W.; Chen, N.; Dong, Z.L.; Hu, M.J.; Qu, L.T. 3D graphene-Fe3O4 nanocomposites with high-performance microwave absorption. Phys. Chem. Chem. Phys. 2013, 15, 13038–13043. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.H.; Wu, H.; Wang, M.L.; Huang, C.S.; Yang, D.P.; Jia, N.Q. Functionalized graphene oxide/Fe3O4 hybrids for cellular magnetic resonance imaging and fluorescence labeling. Mater. Sci. Eng. C-Mater. 2017, 78, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, R.K.; Vaz, A.R.; Savu, R.; Moshkalev, S.A. Self-Assembled and One-Step Synthesis of Interconnected 3D Network of Fe3O4/Reduced Graphene Oxide Nanosheets Hybrid for High-Performance Supercapacitor Electrode. ACS Appl. Mater. Int. 2017, 9, 8880–8890. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gao, M.M.; Yue, W.B.; Jiang, Y.; Wang, Y.; Ren, Y.; Hu, F.Q. Sandwich-Structured Graphene-Fe3O4@Carbon Nanocomposites for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Int. 2015, 7, 9709–9715. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Sun, S. New forms of superparamagnetic nanoparticles for biomedical applications. Adv. Drug Deliv. Rev. 2013, 65, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qi, F.; Zhou, H.; Jia, S.; Gao, Y.; Koh, K.; Yin, Y. Fe3O4@Au nanoparticles as a means of signal enhancement in surface plasmon resonance spectroscopy for thrombin detection. Sens. Actuators B-Chem. 2015, 212, 505–511. [Google Scholar] [CrossRef]
- Li, C.M.; Chen, T.; Ocsoy, I.; Zhu, G.Z.; Yasun, E.; You, M.X.; Wu, C.C.; Zheng, J.; Song, E.Q.; Huang, C.Z.; et al. Gold- Coated Fe3O4 Nanoroses with Five Unique Functions for Cancer Cell Targeting, Imaging, and Therapy. Adv. Funct. Mater. 2014, 24, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Ramay, S.M.; Al-Zaghayer, Y.S.; AlHazaa, A.A.N.; Al Masary, W.A.; Atiq, S. Synthesis and investigation of photocatalytic properties of Au/Fe3O4 nanocomposite materials for degradation of methylene blue. Desalin. Water Treat. 2016, 57, 20069–20075. [Google Scholar] [CrossRef]
- Li, J.C.; Hu, Y.; Yang, J.; Wei, P.; Sun, W.J.; Shen, M.W.; Zhang, G.X.; Shi, X.Y. Hyaluronic acid-modified Fe3O4@Au core/shell nanostars for multimodal imaging and photothermal therapy of tumors. Biomaterials 2015, 38, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.M.; Tavallaie, R.; Sandiford, L.; Tilley, R.D.; Gooding, J.J. Gold coated magnetic nanoparticles: From preparation to surface modification for analytical and biomedical applications. Chem. Commun. 2016, 52, 7528–7540. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Jiao, X.L.; Han, Y.Y.; Jiang, Z.; Chen, D.R. Synthesis of Fe3O4-Au Nanocomposites with Enhanced Peroxidase-Like Activity. Eur. J. Inorg. Chem. 2013, 2013, 109–114. [Google Scholar] [CrossRef]
- Ghorbani, M.; Hamishehkar, H.; Arsalani, N.; Entezami, A.A. Preparation of thermo and pH-responsive polymer@Au/Fe3O4 core/shell nanoparticles as a carrier for delivery of anticancer agent. J. Nanopart. Res. 2015, 17, 305. [Google Scholar] [CrossRef]
- Lo, C.K.; Xiao, D.; Choi, M.M.F. Homocysteine-protected gold-coated magnetic nanoparticles: Synthesis and characterisation. J. Mater. Chem. 2007, 17, 2418. [Google Scholar] [CrossRef]
- Yan, F.; Sun, R. Facile synthesis of bifunctional Fe3O4/Au nanocomposite and their application in catalytic reduction of 4-nitrophenol. Mater. Res. Bull. 2014, 57, 293–299. [Google Scholar] [CrossRef]
- Jin, R.; Song, D.Q.; Xiong, H.X.; Ai, L.S.; Ma, P.Y.; Sun, Y. Magnetic core/shell Fe3O4/Au nanoparticles for studies of quinolones binding to protein by fluorescence spectroscopy. Luminescence 2016, 31, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Viswanathan, S.; Nouws, H.P.; Oliveira, M.B.; Delerue-Matos, C. Iron oxide/gold core/shell nanomagnetic probes and CdS biolabels for amplified electrochemical immunosensing of Salmonella typhimurium. Biosens. Bioelectron. 2014, 51, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Du, Y.M.; Mak, K.Y.; Leung, C.W.; Pong, P.W.T. Novel Hybrid Au/Fe3O4 Magnetic Octahedron-like Nanoparticles with Tunable Size. IEEE Trans. Magn. 2014, 50, 1–5. [Google Scholar]
- Salihov, S.V.; Ivanenkov, Y.A.; Krechetov, S.P.; Veselov, M.S.; Sviridenkova, N.V.; Savchenko, A.G.; Klyachko, N.L.; Golovin, Y.I.; Chufarova, N.V.; Beloglazkina, E.K.; et al. Recent advances in the synthesis of Fe3O4@AU core/shell nanoparticles. J. Magn. Magn. Mater. 2015, 394, 173–178. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wu, L.N.; Wang, F.P.; Jiang, Z.H.; Shen, B.Z. Durian-like multi-functional Fe3O4-Au nanoparticles: Synthesis, characterization and selective detection of benzidine. J. Mater. Chem. A 2013, 1, 9746–9751. [Google Scholar] [CrossRef]
- Li, F.; Yu, Z.F.; Zhao, L.Y.; Xue, T. Synthesis and application of homogeneous Fe3O4 core/Au shell nanoparticles with strong SERS effect. RSC Adv. 2016, 6, 10352–10357. [Google Scholar] [CrossRef]
- Hu, Y.; Meng, L.J.; Niu, L.Y.; Lu, Q.H. Facile Synthesis of Superparamagnetic Fe3O4@polyphosphazene@Au Shells for Magnetic Resonance Imaging and Photothermal Therapy. ACS Appl. Mater. Int. 2013, 5, 4586–4591. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Meng, L.; Lu, Q. Core@shell nanostructures for photothermal conversion: Tunable noble metal nanoshells on cross-linked polymer submicrospheres. J. Mater. Chem. 2010, 20, 5493. [Google Scholar] [CrossRef]
- Ramos-Tejada, M.D.; Viota, J.L.; Rudzka, K.; Delgado, A.V. Preparation of multi-functionalized Fe3O4/Au nanoparticles for medical purposes. Colloid Surf. B. 2015, 128, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Du, J.J.; Jing, C.Y. Preparation of Thiol Modified Fe3O4@Ag Magnetic SERS Probe for PAHs Detection and Identification. J. Phys. Chem. C 2011, 115, 17829–17835. [Google Scholar] [CrossRef]
- Satvekar, R.K.; Tiwari, A.P.; Rohiwal, S.S.; Tiwale, B.M.; Pawar, S.H. A DNA-Assembled Fe3O4@Ag Nanorod in Silica Matrix for Cholesterol Biosensing. J. Mater. Eng. Perform. 2015, 24, 4691–4695. [Google Scholar] [CrossRef]
- Chen, J.Z.; Liu, Y.J.; Zhu, G.X.; Yuan, A.H. Ag@Fe3O4 nanowire: Fabrication, characterization and peroxidase-like activity. Cryst. Res. Technol. 2014, 49, 309–314. [Google Scholar] [CrossRef]
- Gao, G.; Wang, K.; Huang, P.; Zhang, Y.X.; Zhi, X.; Bao, C.C.; Cui, D.X. Superparamagnetic Fe3O4-Ag hybrid nanocrystals as a potential contrast agent for CT imaging. Crystengcomm 2012, 14, 7556–7559. [Google Scholar] [CrossRef]
- Du, J.J.; Cui, J.L.; Jing, C.Y. Rapid in situ identification of arsenic species using a portable Fe3O4@Ag SERS sensor. Chem. Commun. 2014, 50, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Harifi, T.; Montazer, M. Photo-, Bio-, and Magneto-active Colored Polyester Fabric with Hydrophobic/Hydrophilic and Enhanced Mechanical Properties through Synthesis of TiO2/Fe3O4/Ag Nanocomposite. Ind. Eng. Chem. Res. 2014, 53, 1119–1129. [Google Scholar] [CrossRef]
- Xiong, R.; Lu, C.H.; Wang, Y.R.; Zhou, Z.H.; Zhang, X.X. Nanofibrillated cellulose as the support and reductant for the facile synthesis of Fe3O4/Ag nanocomposites with catalytic and antibacterial activity. J. Mater. Chem. A 2013, 1, 14910–14918. [Google Scholar] [CrossRef]
- Wang, L.Y.; Sun, Y.; Wang, J.; Wang, J.A.; Yu, A.M.; Zhang, H.Q.; Song, D.Q. Preparation of surface plasmon resonance biosensor based on magnetic core/shell Fe3O4/SiO2 and Fe3O4/Ag/SiO2 nanoparticles. Colloid Surf. B 2011, 84, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Akhundi, A.; Habibi-Yangjeh, A. High performance magnetically recoverable g-C3N4/Fe3O4/Ag/Ag2SO3 plasmonic photocatalyst for enhanced photocatalytic degradation of water pollutants. Adv. Powder Technol. 2017, 28, 565–574. [Google Scholar] [CrossRef]
- An, Q.; Zhang, P.; Li, J.M.; Ma, W.F.; Guo, J.; Hu, J.; Wang, C.C. Silver-coated magnetite-carbon core-shell microspheres as substrate-enhanced SERS probes for detection of trace persistent organic pollutants. Nanoscale 2012, 4, 5210–5216. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.J.; Chen, J.; Ding, Q.Q.; Lin, D.Y.; Dong, R.L.; Yang, L.B.; Liu, J.H. Sea-urchin-like Fe3O4@C@Ag particles: An efficient SERS substrate for detection of organic pollutants. Nanoscale 2013, 5, 5887–5895. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.X.; Niu, H.L.; Li, P.; Tao, Z.Y.; Mao, C.J.; Song, J.M.; Zhang, S.Y. Multifunctional Fe3O4@C@Ag hybrid nanoparticles: Aqueous solution preparation, characterization and photocatalytic activity. Mater. Res. Bull. 2013, 48, 2415–2419. [Google Scholar] [CrossRef]
- Xia, H.Q.; Cui, B.; Zhou, J.H.; Zhang, L.L.; Zhang, J.; Guo, X.H.; Guo, H.L. Synthesis and characterization of Fe3O4@C@Ag nanocomposites and their antibacterial performance. Appl. Surf. Sci. 2011, 257, 9397–9402. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Z.; Wang, H.B.; Gong, M.; Kong, X.K.; Xia, P.; Chen, Q.W. Multifunctional Fe3O4@C@Ag hybrid nanoparticles as dual modal imaging probes and near-infrared light-responsive drug delivery platform. Biomaterials 2013, 34, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Bisht, G.; Rayamajhi, S.; Biplab, K.C.; Paudel, S.N.; Karna, D.; Shrestha, B.G. Synthesis, Characterization, and Study of In Vitro Cytotoxicity of ZnO-Fe3O4 Magnetic Composite Nanoparticles in Human Breast Cancer Cell Line (MDA-MB-231) and Mouse Fibroblast (NIH 3T3). Nanoscale Res. Lett. 2016, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Saffari, J.; Mir, N.; Ghanbari, D.; Khandan-Barani, K.; Hassanabadi, A.; Hosseini-Tabatabaei, M.R. Sonochemical synthesis of Fe3O4/ZnO magnetic nanocomposites and their application in photo-catalytic degradation of various organic dyes. J. Mater. Sci-Mater. 2015, 26, 9591–9599. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Li, X.; Wang, D.; Wei, B.; Song, H.; Li, X.; Fu, S. Preparation and photocatalytic properties of magnetically reusable Fe3O4@ZnO core/shell nanoparticles. Phys. E 2016, 75, 66–71. [Google Scholar] [CrossRef]
- Shekofteh-Gohari, M.; Habibi-Yangjeh, A. Fe3O4/ZnO/CoWO4 nanocomposites: Novel magnetically separable visible-light-driven photocatalysts with enhanced activity in degradation of different dye pollutants. Ceram. Int. 2017, 43, 3063–3071. [Google Scholar] [CrossRef]
- Chai, L.; Wang, Y.; Zhao, N.; Yang, W.; You, X. Sulfate-doped Fe3O4/Al2O3 nanoparticles as a novel adsorbent for fluoride removal from drinking water. Water Res. 2013, 47, 4040–4049. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Zhang, X.; Liu, Q.; Jing, X.; Liu, J.; Song, D.; Hu, S.; Liu, L.; Wang, J. Synthesis of Fe3O4@TiO2 core–shell magnetic composites for highly efficient sorption of uranium (VI). Colloid Surf. A 2015, 469, 279–286. [Google Scholar] [CrossRef]
- Liu, J.; Xu, J.; Che, R.; Chen, H.; Liu, M.; Liu, Z. Hierarchical Fe3O4@TiO2 yolk-shell microspheres with enhanced microwave-absorption properties. Chemistry 2013, 19, 6746–6752. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Jiang, D.; Yang, S. Enhancement of magnetic properties in hard/soft CoFe2O4/Fe3O4 nanocomposites. RSC Adv. 2016, 6, 46143–46148. [Google Scholar] [CrossRef]
- Fei, C.; Zhang, Y.; Yang, Z.; Liu, Y.; Xiong, R.; Shi, J.; Ruan, X. Synthesis and magnetic properties of hard magnetic (CoFe2O4)–soft magnetic (Fe3O4) nano-composite ceramics by SPS technology. J. Magn. Magn. Mater. 2011, 323, 1811–1816. [Google Scholar] [CrossRef]
- Lavorato, G.; Winkler, E.; Rivas-Murias, B.; Rivadulla, F. Thickness dependence of exchange coupling in epitaxialFe3O4/CoFe2O4soft/hard magnetic bilayers. Phys. Rev. B. 2016, 94, 054405. [Google Scholar] [CrossRef]
- Yi, F. Magnetic properties of hard (CoFe2O4)–soft (Fe3O4) composite ceramics. Ceram. Int. 2014, 40, 7837–7840. [Google Scholar] [CrossRef]
- Shaterabadi, Z.; Nabiyouni, G.; Soleymani, M. High impact of in situ dextran coating on biocompatibility, stability and magnetic properties of iron oxide nanoparticles. Mat. Sci. Eng. C-Mater. 2017, 75, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Osborne, E.A.; Atkins, T.M.; Gilbert, D.A.; Kauzlarich, S.M.; Liu, K.; Louie, A.Y. Rapid microwave-assisted synthesis of dextran-coated iron oxide nanoparticles for magnetic resonance imaging. Nanotechnology 2012, 23, 215602. [Google Scholar] [CrossRef] [PubMed]
- Shete, P.B.; Patil, R.M.; Thorat, N.D.; Prasad, A.; Ningthoujam, R.S.; Ghosh, S.J.; Pawar, S.H. Magnetic chitosan nanocomposite for hyperthermia therapy application: Preparation, characterization and in vitro experiments. Appl. Surf. Sci. 2014, 288, 149–157. [Google Scholar] [CrossRef]
- Mohammadi, A.; Daemi, H.; Barikani, M. Fast removal of malachite green dye using novel superparamagnetic sodium alginate-coated Fe3O4 nanoparticles. Int. J. Biol. Macromol. 2014, 69, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Anbarasu, M.; Anandan, M.; Chinnasamy, E.; Gopinath, V.; Balamurugan, K. Synthesis and characterization of polyethylene glycol (PEG) coated Fe3O4 nanoparticles by chemical co-precipitation method for biomedical applications. Spectrochim. Acta A 2015, 135, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Ardiyanti, H.; Suharyadi, E.; Kato, T.; Iwata, S. Crystal structures and magnetic properties of magnetite (Fe3O4)/polyvinyl alcohol (PVA) ribbon. AIP Conf. Proc. 2016, 1725, 020007. [Google Scholar]
- Ma, Y.R.; Zhang, X.L.; Zeng, T.; Cao, D.; Zhou, Z.; Li, W.H.; Niu, H.Y.; Cai, Y.Q. Polydopamine-Coated Magnetic Nanoparticles for Enrichment and Direct Detection of Small Molecule Pollutants Coupled with MALDI-TOF-MS. ACS Appl. Mater. Int. 2013, 5, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, I.; Aghazadeh, M.; Ganjali, M.R.; Norouzi, P.; Doroudi, T.; Kolivand, P.H. Saccharide-coated superparamagnetic Fe3O4 nanoparticles (SPIONs) for biomedical applications: An efficient and scalable route for preparation and in situ surface coating through cathodic electrochemical deposition (CED). Mater. Lett. 2017, 189, 290–294. [Google Scholar] [CrossRef]
- Calatayud, M.P.; Riggio, C.; Raffa, V.; Sanz, B.; Torres, T.E.; Ibarra, M.R.; Hoskins, C.; Cuschieri, A.; Wang, L.; Pinkernelle, J.; Keilhofff, G.; Goya, G.F. Neuronal cells loaded with PEI-coated Fe3O4 nanoparticles for magnetically guided nerve regeneration. J. Mater. Chem. B 2013, 1, 3607–3616. [Google Scholar] [CrossRef]
- Rose, P.A.; Praseetha, P.K.; Bhagat, M.; Alexander, P.; Abdeen, S.; Chavali, M. Drug Embedded PVP Coated Magnetic Nanoparticles for Targeted Killing of Breast Cancer Cells. Technol. Cancer Res. 2013, 12, 463–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Zhang, B.L.; Wang, J.; Wang, M.; Xie, S.B.; Li, X. Synthesis and characterization of poly(lactic acid)-modified superparamagnetic iron oxide nanoparticles. J. Sol-Gel Sci. Technol. 2016, 77, 335–341. [Google Scholar] [CrossRef]
- Liu, R.; Guo, Y.L.; Odusote, G.; Qu, F.L.; Priestley, R.D. Core-Shell Fe3O4 Polydopamine Nanoparticles Serve Multipurpose as Drug Carrier, Catalyst Support and Carbon Adsorbent. ACS Appl. Mater. Int. 2013, 5, 9167–9171. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.K.; Mathias, R.; Anderson, K.W.; Hilt, J.Z. The effects of synthesis method on the physical and chemical properties of dextran coated iron oxide nanoparticles. Mater. Chem. Phys. 2015, 160, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, C.P.; Zhou, H.P.; Wang, Y.N.; Liu, D.; Li, J.M.; Deng, H.J.; Qi, X.L.; Chen, T.; Zhang, L.M.; Li, G.X. One-pot synthesis of dextran-coated iron oxide nanoclusters for real-time regional lymph node mapping. Int. J. Nanomed. 2017, 12, 3365–3374. [Google Scholar] [CrossRef] [PubMed]
- Unterweger, H.; Tietze, R.; Janko, C.; Zaloga, J.; Lyer, S.; Durr, S.; Taccardi, N.; Goudouri, O.M.; Hoppe, A.; Eberbeck, D.; et al. Development and characterization of magnetic iron oxide nanoparticles with a cisplatin-bearing polymer coating for targeted drug delivery. Int. J. Nanomed. 2014, 9, 3659–3676. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Joshi, N.; Chattopadhyay, K.; De, G. A Facile Synthesis of PEG-Coated Magnetite (Fe3O4) Nanoparticles and Their Prevention of the Reduction of Cytochrome C. ACS Appl. Mater. Int. 2012, 4, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Huang, S.; Yu, K.J.; Clyne, A.M. Dextran and Polymer Polyethylene Glycol (PEG) Coating Reduce Both 5 and 30 nm Iron Oxide Nanoparticle Cytotoxicity in 2D and 3D Cell Culture. Int. J. Mol. Sci. 2012, 13, 5554–5570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thapa, B.; Diaz-Diestra, D.; Beltran-Huarac, J.; Weiner, B.R.; Morell, G. Enhanced MRI T-2 Relaxivity in Contrast-Probed Anchor-Free PEGylated Iron Oxide Nanoparticles. Nanoscale Res. Lett. 2017, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.F.; Wu, W.; Ling, J.J.; Wen, S.; Gu, N.; Zhang, X.Z. Effective PEGylation of Iron Oxide Nanoparticles for High Performance In Vivo Cancer Imaging. Adv. Funct. Mater. 2011, 21, 1498–1504. [Google Scholar] [CrossRef]
- Lee, H.; Lee, E.; Kim, D.K.; Jang, N.K.; Jeong, Y.Y.; Jon, S. Antibiofouling Polymer-Coated Superparamagnetic Iron Oxide Nanoparticles as Potential Magnetic Resonance Contrast Agents for in Vivo Cancer Imaging. J. Am. Chem. Soc. 2006, 128, 7383–7389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.L.; Tu, Z.J.; Zhao, F.Y.; Wang, J. Superparamagnetic iron oxide nanoparticles prepared by using an improved polyol method. Appl. Surf. Sci. 2013, 266, 375–379. [Google Scholar] [CrossRef]
- Honary, S.; Ebrahimi, P.; Rad, H.A.; Asgari, M. Optimization of preparation of chitosan-coated iron oxide nanoparticles for biomedical applications by chemometrics approaches. Int. Nano Lett. 2013, 3, 48–52. [Google Scholar] [CrossRef]
- Tang, S.S.; Du, Q.J.; Liu, T.L.; Tan, L.F.; Niu, M.; Gao, L.; Huang, Z.B.; Fu, C.H.; Ma, T.C.; Meng, X.W.; et al. In Vivo Magnetic Resonance Imaging and Microwave Thermotherapy of Cancer Using Novel Chitosan Microcapsules. Nanoscale Res. Lett. 2016, 11, 334. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Yang, L.; Yang, Z.; Yang, H.; Li, A.; Cheng, R. Preparation of chitosan/poly(acrylic acid) magnetic composite microspheres and applications in the removal of copper(II) ions from aqueous solutions. J. Hazard. Mater. 2012, 229–230, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.B.; Shao, H.H.; Jing, G.L.; Huang, F. PEG-chitosan-coated iron oxide nanoparticles with high saturated magnetization as carriers of 10-hydroxycamptothecin: Preparation, characterization and cytotoxicity studies. Colloid Surf. B. 2013, 102, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H.; Beygzadeh, M. Novel high flux antifouling nanofiltration membranes for dye removal containing carboxymethyl chitosan coated Fe3O4 nanoparticles. Desalination 2014, 349, 145–154. [Google Scholar] [CrossRef]
- Lin, M.F.; Thakur, V.K.; Tan, E.J.; Lee, P.S. Surface functionalization of BaTiO3 nanoparticles and improved electrical properties of BaTiO3/polyvinylidene fluoride composite. RSC Adv. 2011, 1, 576–578. [Google Scholar] [CrossRef]
- Thakur, V.K.; Lin, M.F.; Tan, E.J.; Lee, P.S. Green aqueous modification of fluoropolymers for energy storage applications. J. Mater. Chem. 2012, 22, 5951–5959. [Google Scholar] [CrossRef]
- Thakur, V.K.; Yan, J.; Lin, M.F.; Zhi, C.Y.; Golberg, D.; Bando, Y.; Sim, R.; Lee, P.S. Novel polymer nanocomposites from bioinspired green aqueous functionalization of BNNTs. Polym. Chem. 2012, 3, 962–969. [Google Scholar] [CrossRef]
- Cai, H.D.; An, X.; Cui, J.; Li, J.C.; Wen, S.H.; Li, K.G.; Shen, M.W.; Zheng, L.F.; Zhang, G.X.; Shi, X.Y. Facile Hydrothermal Synthesis and Surface Functionalization of Polyethyleneimine-Coated Iron Oxide Nanoparticles for Biomedical Applications. ACS Appl. Mater. Int. 2013, 5, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.W.; Liu, S.; Chang, L. Synthesis of magnetically recyclable Fe3O4@polydopamine-Pt composites and their application in hydrogenation reactions. J. Mater. Sci. 2016, 51, 3643–3649. [Google Scholar] [CrossRef]
- Gopal, S.V.; Mini, R.; Jothy, V.B.; Joe, I.H. Synthesis and characterization of iron oxide nanoparticles using DMSO as a stabilizer. Mater. Today Proc. 2015, 2, 1051–1055. [Google Scholar] [CrossRef]
- Wang, G.S.; Ma, Y.Y.; Tong, Y.; Dong, X.F. Synthesis, characterization and magnetorheological study of 3-aminopropyltriethoxysilane-modified Fe3O4 nanoparticles. Smart Mater. Struct. 2016, 25, 035028. [Google Scholar] [CrossRef]
- Li, K.G.; Shen, M.W.; Zheng, L.F.; Zhao, J.L.; Quan, Q.M.; Shi, X.Y.; Zhang, G.X. Magnetic resonance imaging of glioma with novel APTS-coated superparamagnetic iron oxide nanoparticles. Nanoscale Res. Lett. 2014, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.E.; Amira, M.F.; Zaghloul, A.A.; Ibrahim, G.A.A. Microwave-enforced sorption of heavy metals from aqueous solutions on the surface of magnetic iron oxide-functionalized-3-aminopropyltriethoxysilane. Chem. Eng. J. 2016, 293, 200–206. [Google Scholar] [CrossRef]
- Li, D.; Jiang, D.; Chen, M.; Xie, J.; Wu, Y.; Dang, S.; Zhang, J. An easy fabrication of monodisperse oleic acid-coated Fe3O4 nanoparticles. Mater. Lett. 2010, 64, 2462–2464. [Google Scholar] [CrossRef]
- Yang, K.; Peng, H.; Wen, Y.; Li, N. Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2010, 256, 3093–3097. [Google Scholar] [CrossRef]
- Shete, P.B.; Patil, R.M.; Tiwale, B.M.; Pawar, S.H. Water dispersible oleic acid-coated Fe3O4 nanoparticles for biomedical applications. J. Magn. Magn. Mater. 2015, 377, 406–410. [Google Scholar] [CrossRef]
- Marinca, T.F.; Chicinas, H.F.; Neamtu, B.V.; Isnard, O.; Pascuta, P.; Lupu, N.; Stoian, G.; Chicinas, I. Mechanosynthesis, structural, thermal and magnetic characteristics of oleic acid coated Fe3O4 nanoparticles. Mater. Chem. Phys. 2016, 171, 336–345. [Google Scholar] [CrossRef]
- Velusamy, P.; Chia-Hung, S.; Shritama, A.; Kumar, G.V.; Jeyanthi, V.; Pandian, K. Synthesis of oleic acid coated iron oxide nanoparticles and its role in anti-biofilm activity against clinical isolates of bacterial pathogens. J. Taiwan Inst. Chem. E 2016, 59, 450–456. [Google Scholar] [CrossRef]
- Jin, Y.J.; Liu, F.; Shan, C.; Tong, M.P.; Hou, Y.L. Efficient bacterial capture with amino acid modified magnetic nanoparticles. Water Res. 2014, 50, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.F.; Tsai, C.C.; Lee, M.Z. Linear birefringence and dichroism in citric acid coated Fe3O4 magnetic nanoparticles. J. Magn. Magn. Mater. 2014, 372, 147–158. [Google Scholar] [CrossRef]
- Mohapatra, S.; Mallick, S.K.; Maiti, T.K.; Ghosh, S.K.; Pramanik, P. Synthesis of highly stable folic acid conjugated magnetite nanoparticles for targeting cancer cells. Nanotechnology 2007, 18, 385102. [Google Scholar] [CrossRef]
- Sreeja, V.; Jayaprabha, K.N.; Joy, P.A. Water-dispersible ascorbic-acid-coated magnetite nanoparticles for contrast enhancement in MRI. Appl. Nanosci. 2014, 5, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, C.; Zhao, Y.; Hu, J.; Shao, D.; Wang, X. Synthesis of water-dispersible Fe3O4@β-cyclodextrin by plasma-induced grafting technique for pollutant treatment. Chem. Eng. J. 2013, 229, 296–303. [Google Scholar] [CrossRef]
- Mumtaz, S.; Wang, S.; Hussain, S.Z.; Abdullah, M.; Huma, Z.; Iqbal, Z.; Creran, B.; Rotello, V.M.; Hussain, I. Dopamine coated Fe3O4 nanoparticles as enzyme mimics for the sensitive detection of bacteria. Chem. Commun. 2017, 53, 12306–12308. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Insin, N.; Lee, J.; Han, H.S.; Cordero, J.M.; Liu, W.H.; Bawendi, M.G. Compact Zwitterion-Coated Iron Oxide Nanoparticles for Biological Applications. Nano Lett. 2012, 12, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Mamani, J.B.; Costa-Filho, A.J.; Cornejo, D.R.; Vieira, E.D.; Gamarra, L.F. Synthesis and characterization of magnetite nanoparticles coated with lauric acid. Mater. Charact. 2013, 81, 28–36. [Google Scholar] [CrossRef]
- Ruiz, A.; Morais, P.C.; de Azevedo, R.B.; Lacava, Z.G.M.; Villanueva, A.; del Puerto Morales, M. Magnetic nanoparticles coated with dimercaptosuccinic acid: Development, characterization, and application in biomedicine. J. Nanopart. Res. 2014, 16. [Google Scholar] [CrossRef]
- Liu, Y.X.; Wang, J.K. Effects of DMSA-Coated Fe3O4 Nanoparticles on the Transcription of Genes Related to Iron and Osmosis Homeostasis. Toxicol. Sci. 2013, 131, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, I.; Aghazadeh, M.; Doroudi, T.; Ganjali, M.R.; Koliv, P.H.; Gharailou, D. Amino Acid Coated Superparamagnetic Iron Oxide Nanoparticles for Biomedical Applications Through a Novel Efficient Preparation Method. J. Clust. Sci. 2017, 28, 1259–1271. [Google Scholar] [CrossRef]
- Sahoo, Y.; Goodarzi, A.; Swihart, M.T.; Ohulchanskyy, Y.T.; Kaur, N.; Furlani, E.P.; Prasad, P.N. Aqueous Ferrofluid of Magnetite Nanoparticles: Fluorescence Labeling and Magnetophoretic Control. J. Phys. Chem. B. 2005, 109, 3879–3885. [Google Scholar] [CrossRef] [PubMed]
- Durdureanu-Angheluta, A.; Mihesan, C.; Doroftei, F.; Dascalu, A.; Ursu, L.; Velegrakis, M.; Pinteala, M. Formation by laser ablation in liquid (LAL) and characterization of citric acid-coated iron oxide nanoparticles. Rev. Roum. Chim. 2014, 59, 151–159. [Google Scholar]
- An, P.; Zuo, F.; Wu, Y.P.; Zhang, J.H.; Zheng, Z.H.; Ding, X.B.; Peng, Y.X. Fast synthesis of dopamine-coated Fe3O4 nanoparticles through ligand-exchange method. Chin. Chem. Lett. 2012, 23, 1099–1102. [Google Scholar] [CrossRef]
- Herranz, F.; Morales, M.P.; Roca, A.G.; Desco, M.; Ruiz-Cabello, J. A new method for the rapid synthesis of water stable superparamagnetic nanoparticles. Chemistry 2008, 14, 9126–9130. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.M.; Shete, P.B.; Thorat, N.D.; Otari, S.V.; Barick, K.C.; Prasad, A.; Ningthoujam, R.S.; Tiwale, B.M.; Pawar, S.H. Non-aqueous to aqueous phase transfer of oleic acid coated iron oxide nanoparticles for hyperthermia application. RSC Adv. 2014, 4, 4515–4522. [Google Scholar] [CrossRef]
- Cai, J.; Miao, Y.Q.; Yu, B.Z.; Ma, P.; Li, L.; Fan, H.M. Large-Scale, Facile Transfer of Oleic Acid-Stabilized Iron Oxide Nanoparticles to the Aqueous Phase for Biological Applications. Langmuir 2017, 33, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Pileni, M.P. Reverse Micelles as Microreactors. J. Phys. Chem. 1993, 97, 6961–6973. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, F.L.; Li, Z.Y.; Zhong, Z.Q.; Govorov, A.; Fu, L.; Tan, H.; Jagadish, C.; Wang, Z.M. Giant optical pathlength enhancement in plasmonic thin film solar cells using core-shell nanoparticles. J. Phys. D Appl. Phys. 2018, 51, 8. [Google Scholar] [CrossRef]











| Method | Advantages | Disadvantages |
|---|---|---|
| Co-precipitation method | Simple and efficient | Size distribution, poor crystallinity and aggregation |
| Hydrothermal reactions | Easy to control particle size and shape | Long reaction time, high reaction temperature, high pressure |
| Thermal decomposition | Good control of size and shapes, high yield | High reaction temperature |
| Microemulsion method | Control of particle size, highly homogeneous | Poor yield, large amounts of solvent required and time consuming |
| Sol-gel reactions | Precise control of size and structure | Relatively expensive, long reaction time |
| Aerosol/vapor phase method | High yield | Extremely high temperatures |
| Electrochemical method | Easy control of size | Reproducibility |
| Synthesis Methods | Advantages | Disadvantages |
|---|---|---|
| Stöber method | Controllable silica shell and uniform size, high crystallinity | Lack of understanding of its kinetics and mechanism |
| Microemulsion | Control of the particle size, high homogeneous | Poor yield, large amounts of solvent required and time consuming |
| Aerosol pyrolysis | Hermetically-coated | Complex experimental conditions |
| Methods based on sodium silicate solution | Control of crystallinity and surface area | Depends on preparation method |
| Polymer | Source/Production/Preparation | Applications |
|---|---|---|
| Polyethylene glycol (PEG) | Produced by the interaction of ethylene oxide with water, ethylene glycol, or ethylene glycol oligomers | Magnetic resonance imaging (MRI) contrast agents for in vivo cancer imaging, biosensors |
| Polyvinylvinyl pyrrolidone (PVP) | Made from the monomer N-vinylpyrrolidone | Targeted killing of breast cancer cells, MRI contrast agents |
| Polyethylenimine (PEI) | Branched PEI: by the ring opening polymerization of aziridine Linear PEI: by post-modification of other polymers like poly(2-oxazolines) or N-substituted polyaziridines | Cancer cell separation, hyperthermia |
| Polyacrylic acids | Polymerization of acrylic acid | Anticancer drug delivery |
| Polyvinyl alcohol (PVA) | Polymerization of vinyl acetate, then saponification of polyvinyl acetate | In vivo imaging, drug delivery, biosensor |
| Polydopamine (PDA) | Formed from dopamine at slightly basic pH | Catalyst and adsorbent, biosensors |
| Dextran | Produced by lactic acid bacteria | In vivo cancer drug carriers, MRI contrast agents |
| Chitosan | Extracted from shellfish or fungi cell wall | Hyperthermia, tissue engineering |
| Starch | Produced by green plants | Contrasting and imaging |
| Alginate | Extracted from brown algae | Drug-targeted controlled release, adsorbent |
| Polyphenol | Found in some common plant foods like cocoa beans, tea and vegetables | Magnetic hyperthermia |
| Flavonoids | Found in some common plant foods like fruits, vegetables, beans and tea | Cell imaging, nano-carrier; nano-drug |
| Amino acids | In nature | Adsorbent, radio-labeling, biosensors and cancer detection |
| Lipids | In nature, animal food and nuts | Gene therapy, dual-modal imaging |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. https://doi.org/10.3390/nano8100810
Zhu N, Ji H, Yu P, Niu J, Farooq MU, Akram MW, Udego IO, Li H, Niu X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials. 2018; 8(10):810. https://doi.org/10.3390/nano8100810
Chicago/Turabian StyleZhu, Nan, Haining Ji, Peng Yu, Jiaqi Niu, M. U. Farooq, M. Waseem Akram, I. O. Udego, Handong Li, and Xiaobin Niu. 2018. "Surface Modification of Magnetic Iron Oxide Nanoparticles" Nanomaterials 8, no. 10: 810. https://doi.org/10.3390/nano8100810