Hybrid CoO Nanowires Coated with Uniform Polypyrrole Nanolayers for High-Performance Energy Storage Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Fabrication
2.2. Characterization
2.3. Electrochemical Measurement
3. Results and Discussion
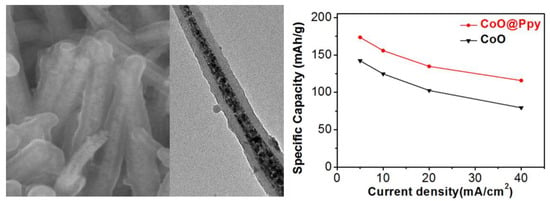
3.1. Structural Characterization of the Electrode
3.2. Electrochemical Properties of the Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yan, J.; Wang, Q.; Wei, T.; Fan, Z. Recent advances in design and fabrication of electrochemical supercapacitors with high energy densities. Adv. Energy Mater. 2014, 4, 1300816. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Sun, W.; Tan, H.T.; Franklin, J.B.; Guo, Y.; Fan, H.; Ulaganathan, M.; Wu, X.-L.; Luo, Z.-Z.; et al. Conversion of uniform graphene oxide/polypyrrole composites into functionalized 3d carbon nanosheet frameworks with superior supercapacitive and sodium-ion storage properties. J. Power Sources 2016, 307, 17–24. [Google Scholar] [CrossRef]
- Yao, X.; Zhao, Y. Three-dimensional porous graphene networks and hybrids for lithium-ion batteries and supercapacitors. Chem 2017, 2, 171–200. [Google Scholar] [CrossRef]
- Huaping, Z.; Long, L.; Ranjith, V.; Yong, L. Recent advances in designing and fabricating self-supported nanoelectrodes for supercapacitors. Adv. Sci. 2017, 4, 1700188. [Google Scholar]
- Zhao, Y.; Wang, L.P.; Sougrati, M.T.; Feng, Z.; Leconte, Y.; Fisher, A.; Srinivasan, M.; Xu, Z. A review on design strategies for carbon based metal oxides and sulfides nanocomposites for high performance li and na ion battery anodes. Adv. Energy Mater. 2016, 6, 1601424. [Google Scholar] [CrossRef]
- Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250–1280. [Google Scholar] [CrossRef]
- Guan, C.; Zhao, W.; Hu, Y.; Lai, Z.; Li, X.; Sun, S.; Zhang, H.; Cheetham, A.K.; Wang, J. Cobalt oxide and n-doped carbon nanosheets derived from a single two-dimensional metal-organic framework precursor and their application in flexible asymmetric supercapacitors. Nanoscale Horiz. 2017, 2, 99–105. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, J.; Fan, Z. Carbon materials for high volumetric performance supercapacitors: Design, progress, challenges and opportunities. Energy Environ. Sci. 2016, 9, 729–762. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Wang, K.; Wu, H.; Meng, Y.; Wei, Z. Conducting polymer nanowire arrays for high performance supercapacitors. Small 2014, 10, 14–31. [Google Scholar]
- Shi, Y.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef]
- Ruffino, F.; Torrisi, V.; Marletta, G.; Grimaldi, M.G. Growth morphology of nanoscale sputter-deposited au films on amorphous soft polymeric substrates. Appl. Phys. A 2011, 103, 939. [Google Scholar] [CrossRef]
- Torrisi, V.; Ruffino, F. Metal-polymer nanocomposites: (co-)evaporation/(co)sputtering approaches and electrical properties. Coatings 2015, 5, 378. [Google Scholar] [CrossRef]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic–inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef]
- Sanchez, C.; Soler-Illia, G.J.D.A.; Ribot, F.; Lalot, T.; Mayer, C.R.; Cabuil, V. Designed hybrid organic−inorganic nanocomposites from functional nanobuilding blocks. Chem. Mater. 2001, 13, 3061–3083. [Google Scholar] [CrossRef]
- Faupel, F.; Zaporojtchenko, V.; Strunskus, T.; Elbahri, M. Metal-polymer nanocomposites for functional applications. Adv. Eng. Mater. 2010, 12, 1177–1190. [Google Scholar] [CrossRef]
- Liu, T.; Finn, L.; Yu, M.; Wang, H.; Zhai, T.; Lu, X.; Tong, Y.; Li, Y. Polyaniline and polypyrrole pseudocapacitor electrodes with excellent cycling stability. Nano Lett. 2014, 14, 2522–2527. [Google Scholar] [CrossRef]
- Xu, J.; Wang, D.; Fan, L.; Yuan, Y.; Wei, W.; Liu, R.; Gu, S.; Xu, W. Fabric electrodes coated with polypyrrole nanorods for flexible supercapacitor application prepared via a reactive self-degraded template. Org. Electron. 2015, 26, 292–299. [Google Scholar] [CrossRef]
- Huang, J.; Wang, K.; Wei, Z. Conducting polymer nanowire arrays with enhanced electrochemical performance. J. Mater. Chem. 2010, 20, 1117–1121. [Google Scholar] [CrossRef]
- Mei, J.; Liao, T.; Kou, L.; Sun, Z. Two-dimensional metal oxide nanomaterials for next-generation rechargeable batteries. Adv. Mater. 2017, 29, 1700176. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xu, Y.; Lei, Y. Heterogeneous nanostructure array for electrochemical energy conversion and storage. Nano Today 2018, 20, 33–57. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, P.; Liang, K.; Yao, M.; Yang, Y.; Hu, W. Cvd-grown polypyrrole nanofilms on highly mesoporous structure mno2 for high performance asymmetric supercapacitors. Chem. Eng. J. 2017, 307, 105–112. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Zhou, L.; Huang, X.; Yu, C. Polypyrrole-coated zinc ferrite hollow spheres with improved cycling stability for lithium-ion batteries. Small 2016, 12, 3732–3737. [Google Scholar] [CrossRef]
- Bhaskar, A.; Deepa, M.; Ramakrishna, M.; Rao, T.N. Poly(3,4-ethylenedioxythiophene) sheath over a SnO2 hollow spheres/graphene oxide hybrid for a durable anode in li-ion batteries. J. Phys. Chem. C 2014, 118, 7296–7306. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.; Mao, J.; Deng, J.; Gao, Q.; Tang, Y.; Schmidt, O.G. Hierarchical mos2/polyaniline nanowires with excellent electrochemical performance for lithium-ion batteries. Adv. Mater. 2012, 25, 1180–1184. [Google Scholar] [CrossRef]
- Guan, C.; Liu, J.; Cheng, C.; Li, H.; Li, X.; Zhou, W.; Zhang, H.; Fan, H.J. Hybrid structure of cobalt monoxide nanowire@nickel hydroxidenitrate nanoflake aligned on nickel foam for high-rate supercapacitor. Energy Environ. Sci. 2011, 4, 4496–4499. [Google Scholar] [CrossRef]
- Guan, C.; Li, X.; Yu, H.; Mao, L.; Wong, L.H.; Yan, Q.; Wang, J. A novel hollowed coo-in-cosno3 nanostructure with enhanced lithium storage capabilities. Nanoscale 2014, 6, 13824–13830. [Google Scholar] [CrossRef]
- Guan, C.; Xia, X.; Meng, N.; Zeng, Z.; Cao, X.; Soci, C.; Zhang, H.; Fan, H.J. Hollow core-shell nanostructure supercapacitor electrodes: Gap matters. Energy Environ. Sci. 2012, 5, 9085–9090. [Google Scholar] [CrossRef]
- Li, X.; Wu, H.; Elshahawy, A.M.; Wang, L.; Pennycook, S.J.; Guan, C.; Wang, J. Cactus-like nicop/nico-oh 3d architecture with tunable composition for high-performance hybrid supercapacitors. Adv. Funct. Mater. 2018, 28, 1800036. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, L.; Xiao, Y.; Shen, L.; Chen, Q.; Shi, W. Hydrogenated coox nanowire @ Ni(OH)2 nanosheet core-shell nanostructures for high-performance asymmetric supercapacitors. Nanoscale 2014, 6, 6772–6781. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-L.; Guo, R.; Ding, L.-X.; Tong, Y.-X.; Li, G.-R. Controllable template-assisted electrodeposition of single- and multi-walled nanotube arrays for electrochemical energy storage. Sci. Rep. 2013, 3, 1204. [Google Scholar] [CrossRef]
- Xu, K.; Huang, X.; Liu, Q.; Zou, R.; Li, W.; Liu, X.; Li, S.; Yang, J.; Hu, J. Understanding the effect of polypyrrole and poly(3,4-ethylenedioxythiophene) on enhancing the supercapacitor performance of nico2o4 electrodes. J. Mater. Chem. A 2014, 2, 16731–16739. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Li, Y.; Liu, J. Construction of high-capacitance 3d coo@polypyrrole nanowire array electrode for aqueous asymmetric supercapacitor. Nano Lett. 2013, 13, 2078–2085. [Google Scholar] [CrossRef]
- Wang, H.; Qing, C.; Guo, J.; Aref, A.A.; Sun, D.; Wang, B.; Tang, Y. Highly conductive carbon-coo hybrid nanostructured arrays with enhanced electrochemical performance for asymmetric supercapacitors. J. Mater. Chem. A 2014, 2, 11776–11783. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J. Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Lyu, F.; Bai, Y.; Li, Z.; Xu, W.; Wang, Q.; Mao, J.; Wang, L.; Zhang, X.; Yin, Y. Self-templated fabrication of coo–moo2 nanocages for enhanced oxygen evolution. Adv. Funct. Mater. 2017, 27, 1702324. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, J.; Zhang, J.; Ma, H.; He, Y.; Li, F.; Xie, E.; Xue, D.; Zhang, H.; Peng, Y. Co@Co3O4 core–shell three-dimensional nano-network for high-performance electrochemical energy storage. Small 2014, 10, 2618–2624. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Chen, H.; Guan, C. Hybrid CoO Nanowires Coated with Uniform Polypyrrole Nanolayers for High-Performance Energy Storage Devices. Nanomaterials 2019, 9, 586. https://doi.org/10.3390/nano9040586
Yang C, Chen H, Guan C. Hybrid CoO Nanowires Coated with Uniform Polypyrrole Nanolayers for High-Performance Energy Storage Devices. Nanomaterials. 2019; 9(4):586. https://doi.org/10.3390/nano9040586
Chicago/Turabian StyleYang, Chunhai, Hao Chen, and Cao Guan. 2019. "Hybrid CoO Nanowires Coated with Uniform Polypyrrole Nanolayers for High-Performance Energy Storage Devices" Nanomaterials 9, no. 4: 586. https://doi.org/10.3390/nano9040586