Properties and Mechanism of Hydroxyapatite Coating Prepared by Electrodeposition on a Braid for Biodegradable Bone Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Preparation of PVA/PLA Braids
2.2. The Electrodeposition of HA Coatings
2.3. The Characterization of HA Coatings
3. Results and Discussion
3.1. The Surface Morphology and Element Composition of HA Coatings
3.2. The Chemical Bonds and Phase Composition of HA Coatings
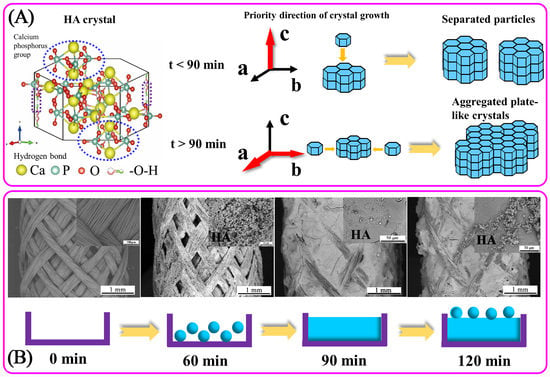
3.3. The Formation Mechanism of HA Coatings Prepared by Electrodeposition
4. Conclusions
- (a)
- With the increase of time, the particles aggregate and extrude each other to form flat-like HA crystals with a smooth and compact plane. The Ca/P ratio of the HA coating gradually increases to 1.34, 1.44, and 1.70 with the increase in deposition time. Roughness of HA coating after 90 min-deposition reaches 0.725 μm, which is 4 times better than that after 60 min deposition.
- (b)
- Crystallinity degree increases from 33.30% to 57.55% when deposition time increases from 60 min to 120 min. Moreover, HA crystal shows a preferred orientation along the c-axis. Correspondingly, the unit cell parameter a (Å)/b (Å) decreases first and then increases with deposition time, while the value of c (Å) increases first and then decreases.
- (c)
- The forming mechanism of HA coating shows as follow. At the initial stage of deposition (<60 min), HA crystals nucleate on the braid surface; after nuclei completely covers with the surface of PVA/PLA braid (>60 min), HA crystals begin to grow, and then aggregated to a smooth plate-like plane; When deposited for a long time (>90 min), circular HA particles are formed on the plate-like plane again, resulting in a slight increase in roughness.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- He, F.; Tian, Y.; Fang, X.; Xu, Y.; Ye, J. Porous calcium phosphate composite bioceramic beads. Ceram. Int. 2018, 44, 13430–13433. [Google Scholar] [CrossRef]
- Nunez, D.; Elgueta, E.; Varaprasad, K.; Oyarzun, P. Hydroxyapatite nanocrystals synthesized from calcium rich bio-wastes. Mater. Lett. 2018, 230, 64–68. [Google Scholar] [CrossRef]
- Rau, J.V.; Cacciotti, I.; Laureti, S.; Fosca, M.; Varvaro, G.; Latini, A. Bioactive, nanostructured Si-substituted hydroxyapatite coatings on titanium prepared by pulsed laser deposition. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I. Multisubstituted hydroxyapatite powders and coatings: The influence of the codoping on the hydroxyapatite performances. Int. J. Appl. Ceram. Technol. 2019. [Google Scholar] [CrossRef]
- Cacciotti, I. Cationic and Anionic Substitutions in Hydroxyapatite. In Handbook of Bioceramics and Biocomposites; Antoniac, I.V., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 145–211. [Google Scholar] [CrossRef]
- Rau, J.V.; Cacciotti, I.; Bonis, A.D.; Fosca, M.; Komlev, V.S.; Latini, A.; Santagata, A.; Teghil, R. Fe-doped hydroxyapatite coatings for orthopedic and dental implant applications. Appl. Surf. Sci. 2014, 307, 301–305. [Google Scholar] [CrossRef]
- Xu, M.; Ma, F.; Liu, P.; Li, W.; Liu, X.; Chen, X.; He, D.; Geng, F. Influences of pH Value and Deposition Time on HA/TiO2 Coatings Deposited by Electrochemical Method. Mater. Trans. 2014, 55, 937–941. [Google Scholar] [CrossRef]
- Mao, Z.-L.; Yang, X.-J.; Zhu, S.-L.; Cui, Z.-D.; Li, Z.-Y. Effect of Na+ and NaOH concentrations on the surface morphology and dissolution behavior of hydroxyapatite. Ceram. Int. 2015, 41, 3461–3468. [Google Scholar] [CrossRef]
- Liu, C.P.; Yang, Y.X. Cytocompatibility Evaluation of Hydroxyapatite Coating on Titanium Surfaces by Pulsed Electrochemical Deposition. Asian J. Chem. 2014, 26, 5466–5468. [Google Scholar] [CrossRef]
- Stango, S.A.X.; Karthick, D.; Swaroop, S.; Mudali, U.K.; Vijayalakshmi, U. Development of hydroxyapatite coatings on laser textured 316 LSS and Ti-6Al-4V and its electrochemical behavior in SBF solution for orthopedic applications. Ceram. Int. 2018, 44, 3149–3160. [Google Scholar] [CrossRef]
- Lou, C.W.; Kuo, S.T.; Wen, S.P.; Lin, J.H. Braided Bone Scaffolds Made by Braiding Polyvinyl Alcohol and Cross-Linked by Glutaraldehyde: Manufacturing Process and Structure Evaluation. Adv. Mater. Res. 2014, 910, 145–148. [Google Scholar] [CrossRef]
- Lin, J.H.; Lee, M.C.; Chen, C.K.; Huang, C.L.; Chen, Y.S.; Wen, S.P.; Kuo, S.T.; Lou, C.W. Recovery evaluation of rats’ damaged tibias: Implantation of core-shell structured bone scaffolds made using hollow braids and a freeze-thawing process. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.; Surmeneva, M.; Grubova, I.; Chernozem, R.; Krause, B.; Baumbach, T.; Loza, K.; Epple, M. RF magnetron sputtering of a hydroxyapatite target: A comparison study on polytetrafluorethylene and titanium substrates. Appl. Surf. Sci. 2017, 414, 335–344. [Google Scholar] [CrossRef]
- Kim, I.Y.; Cho, S.B.; Ohtsuki, C. Coating of hydroxyapatite on cordierite through biomimetic processing. J. Ceram. Process. Res. 2014, 15, 474–479. [Google Scholar]
- Sidane, D.; Chicot, D.; Yala, S.; Ziani, S.; Ithireddine, H.; Lost, A.; Decoopman, X. Study of the mechanical behavior and corrosion resistance of hydroxyapatite sol-gel thin coatings on 316 L stainless steel pre-coated with titania film. Thin Solid Films 2015, 593, 71–80. [Google Scholar] [CrossRef]
- Amirnejad, M.; Afshar, A.; Salehi, S. The Effect of Titanium Dioxide (TiO2) Nanoparticles on Hydroxyapatite (HA)/TiO2 Composite Coating Fabricated by Electrophoretic Deposition (EPD). J. Mater. Eng. Perform. 2018, 27, 2338–2344. [Google Scholar] [CrossRef]
- Hamdi, D.A.; Jiang, Z.-T.; No, K.; Rahman, M.M.; Lee, P.-C.; Truc, L.N.T.; Kim, J.; Altarawneh, M.; Thair, L.; Jumaa, T.A.-J.; et al. Biocompatibility study of multi-layered hydroxyapatite coatings synthesized on Ti-6Al-4V alloys by RF magnetron sputtering for prosthetic-orthopaedic implant applications. Appl. Surf. Sci. 2019, 463, 292–299. [Google Scholar] [CrossRef]
- Asri, R.I.; Harun, W.S.; Hassan, M.A.; Ghani, S.A.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol-gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef]
- Ghani, Y.; Coathup, M.J.; Hing, K.A.; Blunn, G.W. Antibacterial effect of incorporating silver ions in electrochemically deposited hydroxyapatite coating: An experimental study. JRSM Short Rep. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi-Rad, M. Effect of morphology on the electrophoretic deposition of hydroxyapatite nanoparticles. J. Alloys Compd. 2018, 741, 211–222. [Google Scholar] [CrossRef]
- Lin, M.C.; Lou, C.W.; Lin, J.Y.; Lin, T.A.; Chen, Y.S.; Lin, J.H. Biodegradable Polyvinyl Alcohol Vascular Stents: Structural Model and Mechanical and Biological Property Evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 404–413. [Google Scholar] [CrossRef]
- Maheshwari, S.U.; Samuel, V.K.; Nagiah, N. Fabrication and evaluation of (PVA/HAp/PCL) bilayer composites as potential scaffolds for bone tissue regeneration application. Ceram. Int. 2014, 40, 8469–8477. [Google Scholar] [CrossRef]
- Stipniece, L.; Narkevica, I.; Sokolova, M.; Locs, J.; Ozolins, J. Novel scaffolds based on hydroxyapatite/poly(vinyl alcohol) nanocomposite coated porous TiO2 ceramics for bone tissue engineering. Ceram. Int. 2016, 42, 1530–1537. [Google Scholar] [CrossRef]
- Bianco, A.; Cacciotti, I.; Lombardi, M.; Montanaro, L.; Bemporad, E.; Sebastiani, M. F-substituted hydroxyapatite nanopowders: Thermal stability, sintering behaviour and mechanical properties. Ceram. Int. 2010, 36, 313–322. [Google Scholar] [CrossRef]
- Jang, J.-M.; Chung, S.-W.; Choe, H.-C.; Brantley, W.A. Electrochemical deposition behavior and characterization of Pd-Ag-HAp nanoparticles on ultra-fine TiO2 nanotubes. Surf. Coat. Technol. 2017, 320, 383–390. [Google Scholar] [CrossRef]
- Mokabber, T.; Lu, L.Q.; van Rijn, P.; Vakis, A.I.; Pei, Y.T. Crystal growth mechanism of calcium phosphate coatings on titanium by electrochemical deposition. Surf. Coat. Technol. 2018, 334, 526–535. [Google Scholar] [CrossRef]
- Pang, S.M.; He, Y.; He, P.; Luo, X.S.; Guo, Z.Z.; Li, H. Fabrication of two distinct hydroxyapatite coatings and their effects on MC3T3-E1 cell behavior. Colloids Surf. B Biointerfaces 2018, 171, 40–48. [Google Scholar] [CrossRef]
- Chakraborty, R.; Seesala, V.S.; Sengupta, S.; Dhara, S.; Saha, P.; Das, K.; Das, S. Comparison of Osteoconduction, cytocompatibility and corrosion protection performance of hydroxyapatite-calcium hydrogen phosphate composite coating synthesized in-situ through pulsed electro-deposition with varying amount of phase and crystallinity. Surf. Interfaces 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Fathyunes, L.; Khalil-Allafi, J. Effect of employing ultrasonic waves during pulse electrochemical deposition on the characteristics and biocompatibility of calcium phosphate coatings. Ultrason. Sonochem. 2018, 42, 293–302. [Google Scholar] [CrossRef]
- Fathyunes, L.; Khalil-Allafi, J.; Sheykholeslami, S.O.R.; Moosavifar, M. Biocompatibility assessment of graphene oxide-hydroxyapatite coating applied on TiO2 nanotubes by ultrasound-assisted pulse electrodeposition. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 87, 10–21. [Google Scholar] [CrossRef]
- Ramahdita, G.; Puspita, D.M.; Albab, M.F.; Alfata, R.; Sofyan, N.; Yuwono, A.H. The Effect of Hydroxyapatite Addition on the Mechanical Properties of Polyvinyl Alcohol/Chitosan Biomaterials for Bone Scaffolds Application. In 2nd Biomedical Engineerings Recent Progress in Biomaterials, Drugs Development, and Medical Devices; Ramahdita, G., Wulan, P., Dhelika, R., Whulanza, Y., Eds.; Amer Inst Physics: Bali, Indonesia, 2018; Volume 1933. [Google Scholar]
- Rodrigues, B.V.M.; Leite, N.C.S.; Cavalcanti, B.d.N.; da Silva, N.S.; Marciano, F.R.; Corat, E.J.; Webster, T.J.; Lobo, A.O. Graphene oxide/multi-walled carbon nanotubes as nanofeatured scaffolds for the assisted deposition of nanohydroxyapatite: Characterization and biological evaluation. Int. J. Nanomed. 2016, 11, 2569–2585. [Google Scholar] [CrossRef]
- Cacciotti, I.; Mori, S.; Cherubini, V.; Nanni, F. Eco-sustainable systems based on poly(lactic acid), diatomite and coffee grounds extract for food packaging. Int. J. Biol. Macromol. 2018, 112, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Santos, R.; Monreal-Romero, H.; Guadalupe Chacon-Nava, J. PVA/HAp composite with pork bone precursor obtained by electrospinning. Micro Nano Lett. 2017, 12, 321–324. [Google Scholar] [CrossRef]
- Basargan, T.; Erdol-Aydin, N.; Nasun-Saygili, G. Spray dried hydroxyapatite-polyvinyl alcohol biocomposites. J. Polymer Eng. 2016, 36, 795–804. [Google Scholar] [CrossRef]
- Wang, J.; Hidayah, Z.N.; Abd Razak, S.I.; Kadir, M.R.A.; Nayan, N.H.M.; Li, Y.; Amin, K.A.M. Surface entrapment of chitosan on 3D printed polylactic acid scaffold and its biomimetic growth of hydroxyapatite. Compos. Interfaces 2019, 26, 465–478. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, Z.; Zhang, X.; Chang, X.; Zhang, X.; Li, Y.; Ye, T.; Han, R.; Han, S.; Gao, Y.; et al. Nanotube-formed Ti substrates coated with silicate/silver co-doped hydroxyapatite as prospective materials for bone implants. J. Alloys Compd. 2017, 697, 182–199. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, C.; Bao, Y.; Dai, G. Carbon fibers with a nano-hydroxyapatite coating as an excellent biofilm support for bioreactors. Appl. Surf. Sci. 2018, 443, 255–265. [Google Scholar] [CrossRef]
- Li, F.; Jiang, X.; Shao, Z.; Zhu, D.; Luo, Z. Research Progress Regarding Interfacial Characteristics and the Strengthening Mechanisms of Titanium Alloy/Hydroxyapatite Composites. Materials 2018, 11, 1391. [Google Scholar] [CrossRef] [PubMed]
- Vladescu, A.; Vranceanu, D.M.; Kulesza, S.; Ivanov, A.N.; Bramowicz, M.; Fedonnikov, A.S.; Braic, M.; Norkin, I.A.; Koptyug, A.; Kurtukova, M.O.; et al. Influence of the electrolyte’s pH on the properties of electrochemically deposited hydroxyapatite coating on additively manufactured Ti64 alloy. Sci. Rep. 2017, 7, 16819. [Google Scholar] [CrossRef] [Green Version]
- Bianco, A.; Cacciotti, I.; Lombardi, M.; Montanaro, L.; Gusmano, G. Thermal stability and sintering behaviour of hydroxyapatite nanopowders. J. Thermal Anal. Calorim. 2007, 88, 237–243. [Google Scholar] [CrossRef]
- Siddiqi, S.A.; Azhar, U.; Manzoor, F.; Jamal, A.; Tariq, M.; Saleem, M.; Chaudhry, A.A.; Rehman, I.U. Fabrication of Biocompatible Nano-Carbonated Hydroxyapatite/Polymer Spongy Scaffolds. Digest J. Nanomater. Biostruct. 2018, 13, 439–450. [Google Scholar]
- Allenstein, U.; Selle, S.; Tadsen, M.; Patzig, C.; Hoeche, T.; Zink, M.; Mayr, S.G. Coupling of Metals and Biominerals: Characterizing the Interface between Ferromagnetic Shape-Memory Alloys and Hydroxyapatite. ACS Appl. Mater. Interfaces 2015, 7, 15331–15338. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-Y.; Chen, J.-Y.; Wen, S.-Y.; Jian, T.-Y. Growth Mechanism and Structural Correlations of Hydroxyapatites on Surface Functionalized Carbon Fibers. J. Electrochem. Soc. 2012, 159, D31–D35. [Google Scholar] [CrossRef]
- Wei, L.; Yang, H.; Hong, J.; He, Z.; Deng, C. Synthesis and structure properties of Se and Sr co-doped hydroxyapatite and their biocompatibility. J. Mater. Sci. 2018, 54, 2514–2525. [Google Scholar] [CrossRef]
- Stevanovic, M.; Dosic, M.; Jankovic, A.; Kojic, V.; Vukasinovic-Sekulic, M.; Stojanovic, J.; Odovic, J.; Sakasc, M.C.; Rhee, K.Y.; Miskovic-Stankovic, V. Gentamicin-Loaded Bioactive Hydroxyapatite/Chitosan Composite Coating Electrodeposited on Titanium. ACS Biomater. Sci. Eng. 2018, 4, 3994–4007. [Google Scholar] [CrossRef]
- Molaei, A.; Yari, M.; Afshar, M.R. Modification of electrophoretic deposition of chitosan-bioactive glass-hydroxyapatite nanocomposite coatings for orthopedic applications by changing voltage and deposition time. Ceram. Int. 2015, 41, 14537–14544. [Google Scholar] [CrossRef]








| Phase | IR Absorption Bands (cm−1) | Description | Ref. |
|---|---|---|---|
| P-O | 561,602 | bending vibrations (υ4) | [30] |
| 957,103.3 | stretching vibrations (υ3) | ||
| -CH2 | 753 | PVA | [31] |
| CO32− | 870 | carbon dioxide dissolving in water | [32] |
| CH2-OH | 1082 | PVA/PLA, stretching vibrations | [33,34] |
| C-OH | 1185 | PVA, stretching vibrations | [35] |
| C-H | 1,385,145.4 | deformation vibrations | [22] |
| C=O | 1744 | PLA, stretching vibrations | [36] |
| -OH...HO- | 3230~3550 | internal hydroxyl band | [37] |
| 2-Theta (deg) | Grain Size (nm) | Miller Indices (h k l) | Standard 2-Theta (deg) |
|---|---|---|---|
| 22.862 | 9.62 | (1 1 1) | 22.752 |
| 25.863 | 15.42 | (0 0 2) | 25.298 |
| 31.778 | 7.37 | (2 1 1) | 31.839 |
| 39.198 | 7.97 | (1 2 2) | 39.253 |
| Average | 10.10 ± 5.32 | — | — |
| Sample | a (Å) | b (Å) | c (Å) |
|---|---|---|---|
| HA60 | 9.3902 | 9.3902 | 7.0644 |
| HA75 | 9.3531 | 9.3531 | 7.0832 |
| HA90 | 9.2818 | 9.2818 | 7.2429 |
| HA105 | 9.3318 | 9.3318 | 7.1110 |
| HA120 | 9.3799 | 9.3799 | 7.1220 |
| Standard HA | 9.4180 | 9.4180 | 6.8840 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.-T.; Ling, L.; Lin, M.-C.; Jiang, Q.; Lin, Q.; Lin, J.-H.; Lou, C.-W. Properties and Mechanism of Hydroxyapatite Coating Prepared by Electrodeposition on a Braid for Biodegradable Bone Scaffolds. Nanomaterials 2019, 9, 679. https://doi.org/10.3390/nano9050679
Li T-T, Ling L, Lin M-C, Jiang Q, Lin Q, Lin J-H, Lou C-W. Properties and Mechanism of Hydroxyapatite Coating Prepared by Electrodeposition on a Braid for Biodegradable Bone Scaffolds. Nanomaterials. 2019; 9(5):679. https://doi.org/10.3390/nano9050679
Chicago/Turabian StyleLi, Ting-Ting, Lei Ling, Mei-Chen Lin, Qian Jiang, Qi Lin, Jia-Horng Lin, and Ching-Wen Lou. 2019. "Properties and Mechanism of Hydroxyapatite Coating Prepared by Electrodeposition on a Braid for Biodegradable Bone Scaffolds" Nanomaterials 9, no. 5: 679. https://doi.org/10.3390/nano9050679