A Novel in Vivo Model for Assessing the Impact of Geophagic Earth on Iron Status
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Study Design and Assessments
2.2.1. Isolation of Total RNA
2.2.2. DMT-1, DcytB and Ferroportin Gene Expression Analysis
2.2.3. Liver Ferritin and Fe
2.2.4. Electrophoresis and Staining Gels
2.3. Statistical Analysis
3. Results
3.1. Growth Rates, Feed Intakes, Fe Intakes and Hemoglobin (Hb)
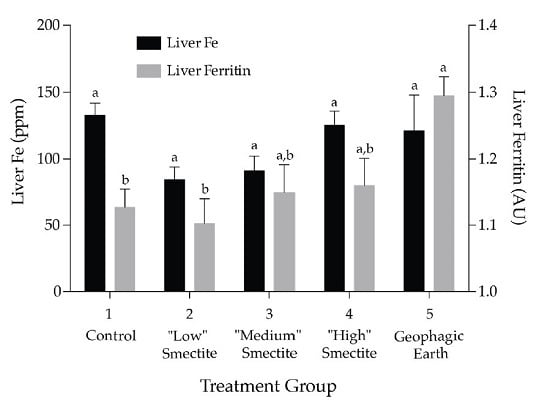
3.2. Liver Ferritin and Fe
3.3. Gene Expression of Fe Transporters DMT-1, Ferroportin and DcytB in the Duodenum
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Fe | iron |
| Hb | hemoglobin |
| TEC | total elemental composition |
| DMT1 | divalent metal transporter 1 |
| DcytB | duodenal cytochrome B |
| CEC | cation exchange capacity |
| GI | gastrointestinal |
| ICP-AES | inductively-coupled plasma atomic emission spectroscopy |
References
- Arnell, B.; Lagercrantz, S. Geophagical Customs; Humanistiska Fonden: Uppsala, Sweden, 1958. [Google Scholar]
- Laufer, B. Geophagy; Field Museum of Natural History: Chicago, IL, USA, 1930. [Google Scholar]
- Young, S.; Sherman, P.; Lucks, J.; Pelto, G. Why on earth? Evaluating hypotheses about the physiological functions of human geophagy. Q. Rev. Biol. 2011, 86, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Wilson, M.; Hillier, S.; Delbos, E. Differences and commonalities in physical, chemical and mineralogical properties of Zanzibari geophagic soils. J. Chem. Biol. 2010, 36, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; Young, S.; Golden, C. A meta-analysis of pica and micronutrient status. Am. J. Hum. Biol. 2015, 27, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Geissler, P.; Shulman, C.; Prince, R. Geophagy, iron status and anemia among pregnant women on the coast of Kenya. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 549–543. [Google Scholar] [CrossRef]
- Thomson, J. Anemia in pregnant women in eastern Caprivi, Namibia. S. Afr. Med. 1997, 87, 1544–1547. [Google Scholar]
- Young, S. Pica in pregnancy: New ideas about an old condition. Annu. Rev. Nutr. 2010, 30, 403–422. [Google Scholar] [CrossRef] [PubMed]
- Adam, I.; Khamis, A.; Elbashir, M. Prevalence and risk factors for anemia in pregnant women of eastern Sudan. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Saathoff, E.; Antelman, G.; Msamanga, G. Geophagy (soil-eating) in relation to anemia and helminth infection among HIV–infected pregnant women in Tanzania. Am. J. Trop. Med. Hyg. 2009, 80, 36–43. [Google Scholar] [PubMed]
- Malenganisho, W.; Magnussen, P. Intake of alcoholic beverages is a predictor of iron status and hemoglobin in adult Tanzanians. J. Nutr. 2007, 137, 2140–2146. [Google Scholar] [PubMed]
- Antelman, G.; Msamanga, G.I.; Spiegelman, D. Nutritional factors and infectious disease contribute to anemia among pregnant women with human immunodeficiency virus in Tanzania. J. Nutr. 2000, 130, 1950–1957. [Google Scholar] [PubMed]
- Tayie, F.; Lartey, A. Pica practice among pregnant Ghanaians: Relationship with infant birth-weight and maternal haemoglobin level. Ghana Med. J. 1999, 33, 67–76. [Google Scholar]
- Aufreiter, S.; Hancock, R.; Mahaney, W. Geochemistry and mineralogy of soils eaten by humans. Int. J. Food Sci. Nutr. 1997, 48, 293–305. [Google Scholar] [CrossRef]
- Hunter, J. Geophagy in Africa and in the United States: A Culture-Nutrition Hypothesis. Geogr. Rev. 1973, 63, 170–195. [Google Scholar] [CrossRef]
- Mahaney, W.; Bezada, M.; Hancock, R. Geophagy of Holstein hybrid cattle in the northern Andes, Venezuela. Mt. Res. Dev. 1996, 16, 177–180. [Google Scholar] [CrossRef]
- Youdim, M.; Iancu, T. Pica hypothesis. Br. J. Haematol. 1977, 36, 298. [Google Scholar] [CrossRef] [PubMed]
- Çavdar, A.; Arcasoy, A. Hematologic and biochemical studies of Turkish children with pica a presumptive explanation for the syndrome of geophagia, iron deficiency anemia, hepatosplenomegaly and hypogonadism. Clin. Pediatr. 1972, 11, 215–223. [Google Scholar] [CrossRef]
- Hooda, P.; Henry, C.; Seyoum, T. The potential impact of soil ingestion on human mineral nutrition. Sci. Total Environ. 2004, 333, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Minnich, V.; Okcuoglu, A.; Tarcon, Y. Pica in Turkey II. Effect of clay upon iron absorption. Am. J. Clin. Nutr. 1968, 21, 78–86. [Google Scholar] [PubMed]
- Sayers, G.; Lipschitz, D.; Sayers, M. Relationship between pica and iron nutrition in Johannesburg Black adults. S. Afr. Med. J. 1974, 48, 1655–1660. [Google Scholar] [PubMed]
- Abrahams, P. Geophagy (soil consumption) and iron supplementation in uganda. Trop. Med. Int. Health 1997, 2, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, P.; Parsons, J. Geophagy in the tropics: An appraisal of three geophagical materials. Environ. Geochem. Health 1997, 19. [Google Scholar] [CrossRef]
- Hunter, J.; DeKleine, R. Geophagy in Central America. Geogr. Rev. 1984, 74, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M. Clay mineralogical and related characteristics of geophagic materials. J. Chem. Ecol. 2003, 29, 1525–1547. [Google Scholar] [CrossRef] [PubMed]
- Benito, P.; Miller, D. Iron absorption and bioavailability: An updated review. Nutr. Res. 1998, 18, 581–603. [Google Scholar] [CrossRef]
- Ruby, M.; Davis, A.; Schoof, R.; Eberle, S. Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environ. Sci. Technol. 1996, 30, 422–430. [Google Scholar] [CrossRef]
- Dreyer, M.; Chaushev, P. Biochemical investigations in geophagia. J. R. Soc. Med. 2004, 97, 48. [Google Scholar] [CrossRef] [PubMed]
- Kikouama, J.; Cornec, F.; Bouttier, S. Evaluation of trace elements released by edible clays in physicochemically simulated physiological media. Int. J. Food Sci. Nutr. 2009, 60, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Rawlins, B.; Cordeiro, M. The bioaccessibility of essential and potentially toxic trace elements in tropical soils from Mukono District, uganda. J. Geol. Soc. Lond. 2000, 157, 885–891. [Google Scholar] [CrossRef]
- Abrahams, P.; Follansbee, M.; Hunt, A.; Smith, B. Iron nutrition and possible lead toxicity: An appraisal of geophagy undertaken by pregnant women of UK Asian communities. Appl. Geochem. 2006, 21, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Pebsworth, P.; Seim, G.; Huffman, M. Soil consumed by Chacma baboons is low in bioavailable iron and high in clay. J. Chem. Ecol. 2013, 39, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Seim, G.; Ahn, C.; Bodis, M.; Luwedde, F.; Miller, D. Bioavailability of iron in geophagic earths and clay minerals, and their effect on dietary iron absorption using an in vitro digestion/Caco-2 cell model. Food Funct. 2013, 4, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Arcasoy, A.; Cavdar, A.O.; Babacan, E. Decreased iron and zinc absorption in Turkish children with iron deficiency and geophagia. Acta Haematol. 1978, 60, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Talkington, K.; Gant, N.F., Jr.; Scott, D. Effect of ingestion of starch and some clays on iron absorption. Am. J. Obstet. Gynecol. 1970, 108, 262–267. [Google Scholar] [CrossRef]
- Tako, E.; Blair, M.; Glahn, R. Biofortified red mottled beans (Phaseolus vulgaris L.) in a maize and bean diet provide more bioavailable iron than standard red mottled beans: Studies in poultry (Gallus gallus) and an in vitro digestion/Caco-2 model. Nutr. J. 2011, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Tako, E.; Glahn, R. White beans provide more bioavailable iron than red beans: studies in poultry (Gallus gallus) and an in vitro digestion/Caco-2 model. Int. J. Vitam. Nutr. Res. 2010, 80, 416. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov: A service of the U.S. National Institutes of Health. Available online: https://clinicaltrials.gov/ (accessed on 9 June 2016).
- Young, S. Craving Earth: Understanding Pica, the Urge to Eat Clay, Starch, Ice, and Chalk; Columbia University Press: New York, NY, USA, 2011. [Google Scholar]
- Tako, E.; Ferket, P.; Uni, Z. Changes in chicken intestinal zinc exporter mRNA expression and small intestinal functionality following intra-amniotic zinc-methionine administration. J. Nutr. Biochem. 2005, 16, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Mete, A.; Zeeland, Y.; Vaandrager, A. Partial purification and characterization of ferritin from the liver and intestinal mucosa of chickens, turtledoves and mynahs. Avian Pathol. 2005, 34, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Passaniti, A.; Roth, T. Purification of chicken liver ferritin by two novel methods and structural comparison with horse spleen ferritin. Biochem. J. 1989, 258, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Leong, L.; Tan, B.; Ho, K. A specific stain for the detection of nonheme iron proteins in polyacrylamide gels. Anal. Biochem. 1992, 207, 317–320. [Google Scholar] [CrossRef]
- Sheng, G.; Johnston, C.; Teppen, B. Adsorption of dinitrophenol herbicides from water by montmorillonites. Clays Clay Miner. 2002, 50, 25–34. [Google Scholar] [CrossRef]
- Johns, T.; Duquette, M. Detoxification and mineral supplementation as functions of geophagy. Am. J. Clin. Nutr. 1991, 53, 448–456. [Google Scholar] [PubMed]
- Fioramonti, J.; Fargeas, M.; Bueno, L. Action of T-2 toxin on gastrointestinal transit in mice: Protective effect of an argillaceous compound. Toxicol. Lett. 1987, 36, 227–232. [Google Scholar] [CrossRef]
- Phillips, T.; Afriyie-Gyawu, E.; Williams, J. Reducing human exposure to aflatoxin through the use of clay: A review. Food Addit. Contam. 2008, 25, 134–145. [Google Scholar] [CrossRef] [PubMed]
- More, J.; Benazet, F.; Fioramonti, J. Effects of treatment with smectite on gastric and intestinal glycoproteins in the rat: A histochemical study. Histochem. J. 1987, 19, 665–670. [Google Scholar] [CrossRef] [PubMed]


| Ingredient | (g/kg Diet) |
|---|---|
| Ground yellow corn | 750 |
| Dry skim milk | 100 |
| DL-Methionine | 2.5 |
| Vegetable oil | 30 |
| Corn starch | 46.5 |
| Choline chloride | 0.75 |
| Vitamin premix a | 10 |
| Mineral premix a | 60 |
| Ferric citrate b | 0.25 |
| Total | 1000 |
| Treatment Group | Dosages g of Clay Minerals or Geophagic Earth Gavaged/Day (mg Fe Content) | |||||
|---|---|---|---|---|---|---|
| 1 (n = 12) | 2 (n = 12) | 3 (n = 12) | 4 (n = 12) | 5 (n = 12) | ||
| Week | Predicted Mean Chicken Mass (kg) | Control a | “Low” Smectite | “Medium” Smectite | “High” Smectite | Geophagic Earth |
| 1 | 0.20 | 0 | 0.04 (19) | 0.07 (34) | 0.10 (48) | 0.20 (3.50) |
| 2 | 0.30 | 0 | 0.06 (29) | 0.10 (48) | 0.15 (72) | 0.30 (5.24) |
| 3 | 0.30 | 0 | 0.06 (29) | 0.10 (48) | 0.15 (72) | 0.30 (5.24) |
| 4 | 0.35 | 0 | 0.07 (34) | 0.12 (58) | 0.17 (82) | 0.35 (6.12) |
| Treatment | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | |
|---|---|---|---|---|---|---|
| Body weight (g) 1 | ||||||
| 1 | Control | 210 a | 243.1 a | 354.0 a | 453.4 a | 520.4 a |
| 2 | Low Smectite | 209 a | 241.6 a | 361.1 a | 480.0 a | 534.2 a |
| 3 | Medium Smectite | 210 a | 233.6 a | 344.6 a | 443.0 a | 494.7 a |
| 4 | High Smectite | 210 a | 232.3 a | 342.2 a | 419.8 a | 458.8 a |
| 5 | Geophagic Earth | 211 a | 250.8 a | 343.4 a | 441.2 a | 503.5 a |
| Feed intake (g/week/bird) 1,2 | ||||||
| 1 | Control | - | 203.4 a | 293.9 a | 346.3 a | 438.8 a |
| 2 | Low Smectite | - | 189.3 a | 276.6 a | 362.9 a | 382.3 a |
| 3 | Medium Smectite | - | 183.7 a | 261.1 a | 308.9 a | 392.3 a |
| 4 | High Smectite | - | 183.2 a | 268.7 a | 324.9 a | 361.8 a |
| 5 | Geophagic Earth | - | 181.9 a | 279.9 a | 307.4 a | 380.2 a |
| Fe intake (mg/week/bird) 1,2,3 | ||||||
| 1 | Control | - | 10.2 a | 14.7 a | 17.3 a | 21.9 a |
| 2 | Low Smectite | - | 10.8 a | 15.8 a | 20.1 a | 21.4 a |
| 3 | Medium Smectite | - | 11.5 a | 16.5 a | 19.5 a | 23.7 a |
| 4 | High Smectite | - | 12.4 a | 18.1 a | 20.9 a | 23.9 a |
| 5 | Geophagic Earth | - | 33.6 b | 50.7 b | 52.1 b | 61.8 b |
| Haemoglobin (g/dL) 4 | ||||||
| 1 | Control | 9.02 a | 8.48 a | 8.37 a | 8.30 a | 8.28 a |
| 2 | Low Smectite | 9.02 a | 8.55 a | 8.40 a | 8.21 a | 8.16 a |
| 3 | Medium Smectite | 9.02 a | 8.43 a | 8.28 a | 8.25 a | 8.18 a |
| 4 | High Smectite | 9.02 a | 8.42 a | 8.24 a | 8.20 a | 8.18 a |
| 5 | Geophagic Earth | 9.02 a | 8.44 a | 8.37 a | 8.30 a | 8.19 a |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seim, G.L.; Tako, E.; Ahn, C.; Glahn, R.P.; Young, S.L. A Novel in Vivo Model for Assessing the Impact of Geophagic Earth on Iron Status. Nutrients 2016, 8, 362. https://doi.org/10.3390/nu8060362
Seim GL, Tako E, Ahn C, Glahn RP, Young SL. A Novel in Vivo Model for Assessing the Impact of Geophagic Earth on Iron Status. Nutrients. 2016; 8(6):362. https://doi.org/10.3390/nu8060362
Chicago/Turabian StyleSeim, Gretchen L., Elad Tako, Cedric Ahn, Raymond P. Glahn, and Sera L. Young. 2016. "A Novel in Vivo Model for Assessing the Impact of Geophagic Earth on Iron Status" Nutrients 8, no. 6: 362. https://doi.org/10.3390/nu8060362