Mass-Production and Characterizations of Polyvinyl Alcohol/Sodium Alginate/Graphene Porous Nanofiber Membranes Using Needleless Dynamic Linear Electrospinning
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
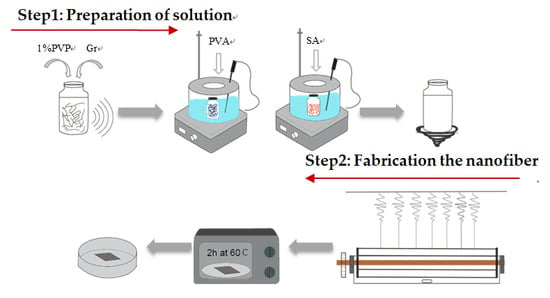
2.2. Formulation of Gr and Gr-AP Suspensions
2.3. Preparation of Linear Electrospinning Nanofiber Membranes
2.4. Measurements and Characterizations
3. Results and Discussion
3.1. Effect of Gr Content, Collection Distance and Voltage on Morphology and Diameter of Gr-AP Nanofiber Membranes
3.2. Far Infrared Ray Spectrum of Gr-AP Nanofiber Membranes
3.3. X-Ray Diffraction of Gr-AP Nanofiber Membranes
3.4. Electrical Properties of Gr-AP Nanofiber Membranes
3.5. Water Contact Angle of Gr-AP Nanofiber Membranes
3.6. Thermal Properties of Gr-AP Nanofiber Membranes
3.7. BET Specific Surface Area of Gr-AP Nanofiber Membranes
4. Conclusions
Author Contributions
Founding
Conflicts of Interest
References
- Ficke, B.W.; Chaudhari, N.M. Nerve Injury. In Orthopedic Surgery Clerkship; Eltorai, A., Eberson, C., Daniels, A., Eds.; Springer: New York, NY, USA, 2017; pp. 302–306. [Google Scholar]
- Isaacs, J. Major peripheral nerve injuries. Hand Clin. 2013, 29, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Neufurth, M.; Ackermann, M.; Tolba, E.; Wang, S.; Feng, Q.; Schröder, H.C.; Wang, X. Fabrication of a new physiological macroporous hybrid biomaterial/bioscaffold material based on polyphosphate and collagen by freeze-extraction. J. Mater. Chem. B 2017, 5, 3823–3835. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Nectow, A.R.; Marra, K.G.; Kaplan, D.L. Biomaterials for the development of peripheral nerve guidance conduits. Tissue Eng. Part B Rev. 2012, 18, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly(lactic acid) nanofibrous scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shera, S.S.; Sahu, S.; Banik, R.M. Preparation of drug eluting natural composite scaffold using response surface methodology and artificial neural network approach. Tissue Eng. Regen. Med. 2018, 15, 1–13. [Google Scholar] [CrossRef]
- Zhuravleva, M.; Gilazieva, Z.; Grigoriev, T.E.; Shepelev, A.D.; Tenchurin, T.K.; Kamyshinsky, R.; Krasheninnikov, S.V.; Orlov, S.; Caralogli, G.; Archipova, S.; et al. In vitro assessment of electrospun polyamide-6 scaffolds for esophageal tissue engineering. J. Biomed. Mater. Res. B 2018. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Persano, L.; Camposeo, A.; Jang, J.; Koo, W.; Kim, S.J.; Cho, H.J.; Kim, I.D.; Pisignano, D. Electrospun nanostructures for high performance chemiresistive and optical sensors. Macromol. Mater. Eng. 2017, 302, 1–37. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, H.; Wang, X. Research and development of aligned nanofibers prepared by electrospinning. J. Text. Res. 2016, 37, 159–166. [Google Scholar]
- Teimouri, A.; Ghorbanian, L.; Dabirian, I. Preparation and characterization of Silk/Diopside composite nanofibers via electrospinning for tissue engineering application. Tectonophysics 2016, 446, 77–96. [Google Scholar]
- Zhang, B.; Kang, F.; Tarascon, J.M.; Kim, J.K. Recent advances in electrospun carbon nanofibers and their application in electrochemical energy storage. Prog. Mater. Sci. 2016, 76, 319–380. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, Z.; Wang, Y.; Shang, X.; Nie, P.; Liu, J. Electrospun polyacrylonitrile/β-cyclodextrin based porous carbon nanofiber self-supporting electrode for capacitive deionization. RSC Adv. 2017, 7, 55224–55231. [Google Scholar] [CrossRef] [Green Version]
- Kaassis, A.Y.; Young, N.; Sano, N.; Merchant, H.A.; Yu, D.G.; Chatterton, N.P.; Williams, G.R. Pulsatile drug release from electrospun poly(ethylene oxide)-sodium alginate blend nanofibers. J. Mater. Chem. B 2014, 2, 1400–1407. [Google Scholar] [CrossRef]
- Min, S.K.; Kim, G.H. Three-dimensional electrospun polycaprolactone (PCL)/alginate hybrid composite scaffolds. Carbohydr. Polym. 2014, 114, 213–221. [Google Scholar]
- Yang, J.M.; Yang, J.H.; Tsou, S.C.; Ding, C.H.; Hsu, C.C.; Yang, K.C.; Yang, C.C.; Chen, K.S.; Chen, S.W.; Wang, J.S. Cell proliferation on PVA/sodium alginate and PVA/poly(γ-glutamic acid) electrospun fiber. Mater. Sci. Eng. C 2016, 66, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.M.; Wu, Q.K. Application progress of graphene, graphene derivatives and graphene composites in tissue engineering. J. Shanghai Jiaotong Univ. 2017, 37, 110–113. [Google Scholar]
- Díezpascual, A.M.; Díezvicente, A.L. Poly(propylene fumarate)/polyethylene glycol-modified graphene oxide nanocomposites for tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 17902–17914. [Google Scholar] [CrossRef] [PubMed]
- Golafshan, N.; Kharaziha, M.; Fathi, M. Tough and conductive hybrid graphene-PVA: Alginate fibrous scaffolds for engineering neural construct. Carbon 2017, 111, 752–763. [Google Scholar] [CrossRef]
- Li, C.; Hou, T.; She, X.; Wei, X.; She, F.; Gao, W.; Kong, L. Decomposition properties of PVA/graphene composites during melting-crystallization. Polym. Degrad. Stab. 2015, 119, 178–189. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Pourbadiei, B.; Doroudian, M.; Azari, S. Preparation of PVA nanocomposites using salep-reduced graphene oxide with enhanced mechanical and biological properties. RSC Adv. 2015, 5, 92428–92437. [Google Scholar] [CrossRef]
- Shadjou, N.; Hasanzadeh, M. Graphene and its nanostructure derivatives for use in bone tissue engineering: Recent advances. J. Biomed. Mater. Res. Part A 2016, 104, 1250–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cengiz-Çallıoǧlu, F.; Jirsak, O.; Dayik, M. Investigation into the relationships between independent and dependent parameters in roller electrospinning of polyurethane. Text. Res. J. 2013, 83, 718–729. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, S.; Qin, X. High throughput of quality nanofibers via one stepped pyramid-shaped spinneret. Mater. Lett. 2013, 106, 56–58. [Google Scholar] [CrossRef]
- Ramalingam, K.J.; Dhineshbabu, N.R.; Srither, S.R.; Saravanakumar, B.; Yuvakkumar, R.; Rajendran, V. Electrical measurement of PVA/graphene nanofibers for transparent electrode applications. Synth. Met. 2014, 191, 113–119. [Google Scholar] [CrossRef]
- Wang, X.; Niu, H.; Wang, X.; Lin, T. Needleless electrospinning of uniform nanofibers using spiral coil spinnerets. J. Nanomater. 2012, 2012, 785920. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Qiu, X.; Huang, X.; Wu, D.; Sun, D. An improved tip-less electrospinning with strip-distributed solution delivery for massive production of uniform polymer nanofibers. Mater. Lett. 2013, 99, 21–23. [Google Scholar] [CrossRef]
- Yan, G.; Niu, H.; Zhou, H.; Wang, H.; Shao, H.; Zhao, X.; Lin, T. Electro-aerodynamic field aided needleless electrospinning. Nanotechnology 2018, 29, 235302. [Google Scholar] [CrossRef] [PubMed]
- Yian, L.; Li, J.; Pan, Z. Research status of multi-jet electrospinning technology. J. Text. Res. 2013, 34, 150–156. [Google Scholar]
- Liu, Y.; He, J.H.; Yu, J.Y. Bubble-electrospinning: A novel method for making nanofibers. J. Phys. Conf. Ser. 2008, 96, 012001. [Google Scholar] [CrossRef]
- He, J.H.; Liu, Y.; Xu, L.; Yu, J.Y.; Sun, G. BioMimic fabrication of electrospun nanofibers with high-throughput. Chaos Solitons Fractals 2008, 37, 643–651. [Google Scholar] [CrossRef]
- Yarin, A.L.; Zussman, E. Upward needleless electrospinning of multiple nanofibers. Polymer 2004, 45, 2977–2980. [Google Scholar] [CrossRef]
- Wang, X.; Niu, H.; Lin, T.; Wang, X. Needleless electrospinning of nanofibers with a conical wire coil. Polym. Eng. Sci. 2010, 49, 1582–1586. [Google Scholar] [CrossRef]
- Senturk-Ozer, S.; Aktas, S.; He, J.; Fisher, F.T.; Kalyon, D.M. Nanoporous nanocomposite membranes via hybrid twin-screw extrusion-multijet electrospinning. Nanotechnology 2017, 28, 025301. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, P.; Ouyang, Z.; Zhang, M.; Lin, Z.; Li, J. Nanoscale graphene doped with highly dispersed silver nanoparticles: Quick synthesis, facile fabrication of 3D membrane-modified electrode, and super performance for electrochemical sensing. Adv. Funct. Mater. 2016, 26, 2122–2134. [Google Scholar] [CrossRef]
- Su, Z.; Li, J.; Li, Q.; Ni, T.; Wei, G. Chain conformation, crystallization behavior, electrical and mechanical properties of electrospun polymer-carbon nanotube hybrid nanofibers with different orientations. Carbon 2012, 50, 5605–5617. [Google Scholar] [CrossRef]
- Li, T.T.; Yan, M.; Wu, Z.H. The spinnability study of the dynamic linear electrode electrospun PVA nanofibers. Mater. Rev. 2018, 24. in press (In Chinese) [Google Scholar]
- Lin, J.H.; Lou, C.W.; Jiang, K.J. Electrospinning Group. Patent CN205152410U, 13 April 2016. [Google Scholar]
- Rutledge, G.C.; Fridrikh, S.V. Formation of fibers by electrospinning. Adv. Drug Deliv. Rev. 2007, 59, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lei, P.; Shan, Y.; Zhang, D. Preparation and characterization of antibacterial electrospun chitosan/poly(vinyl alcohol)/graphene oxide composite nanofibrous membrane. Appl. Surf. Sci. 2018, 435, 832–840. [Google Scholar] [CrossRef]
- Noori, M.; Ravari, F.; Ehsani, M. Preparation of PVA nanofibers reinforced with magnetic graphene by electrospinning method and investigation of their degradation kinetics using master plot analyses on solid state. J. Therm. Anal. Calorim. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Zhou, T.N. The preparation of the poly(vinyl alcohol)/graphene nanocomposites with low percolation threshold and high electrical conductivity by using the large-area reduced graphene oxide sheets. Express Polym. Lett. 2013, 7, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Bao, W.S.; Meguid, S.A.; Zhu, Z.H.; Weng, G.J. Tunneling resistance and its effect on the electrical conductivity of carbon nanotube nanocomposites. J. Appl. Phys. 2012, 111, 093726. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Xu, C.; Zhang, M.; Shang, X. Preparation of graphene/poly(vinyl alcohol) nanocomposites with enhanced mechanical properties and water resistance. Polym. Int. 2011, 60, 816–822. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, L.; Zhao, K.; Wu, Z.; Wang, Y.; Tan, Q. Fabrication of PLGA/HA (core)-collagen/amoxicillin (shell) nanofiber membranes through coaxial electrospinning for guided tissue regeneration. Compos. Sci. Technol. 2016, 125, 100–107. [Google Scholar] [CrossRef]
- He, M.; Xue, J.; Geng, H.; Gu, H.; Chen, D.; Shi, R.; Zhang, L. Fibrous guided tissue regeneration membrane loaded with anti-inflammatory agent prepared by coaxial electrospinning for the purpose of controlled release. Appl. Surf. Sci. 2015, 335, 121–129. [Google Scholar] [CrossRef]
- Vickery, J.L.; Patil, A.J.; Mann, S. Fabrication of graphene-polymer nanocomposites with higher-order three-dimensional architectures. Adv. Mater. 2010, 21, 2180–2184. [Google Scholar] [CrossRef]
- Covelo, A.; Gómez, K.K.; Corona-Lira, P.; Ramírez-Reivich, A.C.; Hernández, M. Electrochemical characterization of PVA/SA nanofibers obtained by electrospinning processing. Surf. Interface Anal. 2018. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.L.; Guo, J.L. Modification of the micro-interface adsorption model on particles with fractal theory-langmuir, freundlich and surface complexation adsorption model. Acta Sci. Circumst. 2005, 25, 52–57. [Google Scholar]
- Kawamura, Y.; Yoshida, H.; Asai, S.; Tanibe, H. Breakthrough curve for adsorption of mercury (II) on polyaminated highly porous chitosan beads. Water Sci. Technol. 1997, 35, 97–105. [Google Scholar] [CrossRef]













| Gr Content (wt.%) | 0 | 0.0375 | 0.075 | 0.25 | 0.50 | 0.75 |
|---|---|---|---|---|---|---|
| Viscosity (±0.1 mPa·s) | 331 | 320 | 340 | 346.2 | 348.9 | 397 |
| Conductivity (±0.01 μS·cm−1) | 865 | 795 | 818 | 858 | 878 | 900 |
| Samples | Melting Point (°C) | T90% (°C) | Mass Change at the First Stage (%) | Mass Change at the Second Stage (%) | TG Residual Mass (%) |
|---|---|---|---|---|---|
| AP | 227.1 | 253.0 | 72.55 | 13.28 | 12.5 |
| 0.0375Gr-AP | 227.9 | 254.9 | 63.26 | 20.38 | 14.07 |
| 0.075Gr-AP | 227.7 | 255.3 | 63.89 | 21.16 | 14.42 |
| 0.25Gr-AP | 226.4 | 254.1 | 62.89 | 20.76 | 14.84 |
| 0.5Gr-AP | 225.8 | 253.3 | 59.88 | 19.30 | 19.70 |
| 0.75Gr-AP | 225.7 | 253.2 | 58.02 | 18.24 | 22.89 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.-T.; Yan, M.; Xu, W.; Shiu, B.-C.; Lou, C.-W.; Lin, J.-H. Mass-Production and Characterizations of Polyvinyl Alcohol/Sodium Alginate/Graphene Porous Nanofiber Membranes Using Needleless Dynamic Linear Electrospinning. Polymers 2018, 10, 1167. https://doi.org/10.3390/polym10101167
Li T-T, Yan M, Xu W, Shiu B-C, Lou C-W, Lin J-H. Mass-Production and Characterizations of Polyvinyl Alcohol/Sodium Alginate/Graphene Porous Nanofiber Membranes Using Needleless Dynamic Linear Electrospinning. Polymers. 2018; 10(10):1167. https://doi.org/10.3390/polym10101167
Chicago/Turabian StyleLi, Ting-Ting, Mengxue Yan, Wenting Xu, Bing-Chiuan Shiu, Ching-Wen Lou, and Jia-Horng Lin. 2018. "Mass-Production and Characterizations of Polyvinyl Alcohol/Sodium Alginate/Graphene Porous Nanofiber Membranes Using Needleless Dynamic Linear Electrospinning" Polymers 10, no. 10: 1167. https://doi.org/10.3390/polym10101167