Fabrication and Characterization of Flexible Medical-Grade TPU Filament for Fused Deposition Modeling 3DP Technology
Abstract
:1. Introduction
2. Experimental
2.1. S-TPU Synthesis
2.2. Identification of Free Isocyanate Groups
- % NCO–percentage of unbound isocyanate groups (% mass)
- V0—volume of HCl solution used for blank probe titration (cm3)
- V1—volume of HCl solution used for the test sample titration (cm3)
- m—sample mass (g]
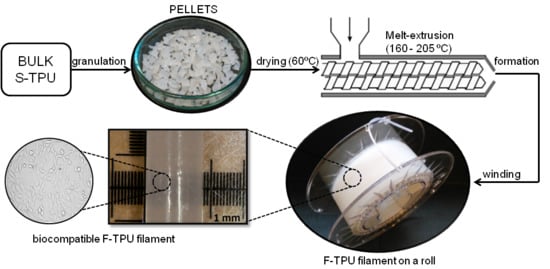
2.3. Melt-Extrusion of F-TPU Filament
2.4. Material Characterization Techniques
2.4.1. Density
2.4.2. Melt Flow Rate (MFR)
2.4.3. Mechanical Characterization
2.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.5. Optical Microscopy (OM)
2.4.6. Contact Angle (CA)
2.5. Biological Characterization
2.5.1. Short-Term Hemocompatibility Test
2.5.2. Indirect Cytotoxicity Test
2.5.3. Statistical Analysis
2.5.4. Analysis Cells Morphology
3. Results and Discussions
3.1. S-TPU Synthesis—Identification of Free Isocyanate Groups
3.2. Fabrication of F-TPU Filament from Synthesized S-TPU Granules
3.3. Physico-Mechanical Properties of Synthesized S-TPU
3.4. The Impact Assessment of Filament Formation on Selected S-TPU Properties
3.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.4.2. Optical Microscopy (OM)
3.4.3. Water Contact Angle (CA)
3.5. Biological Studies
3.5.1. Short-Term Hemocompatibility Test
3.5.2. Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Javaid, M.; Haleem, A. Additive manufacturing applications in medical cases: A literature based review. Alexandria J. Med. 2017. [Google Scholar] [CrossRef]
- Schubert, C.; Van Langeveld, M.C.; Donoso, L.A. Innovations in 3D printing: A 3D overview from optics to organs. Br. J. Ophthalmol. 2014, 98, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Medical Applications for 3D Printing: Current and Projected Uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar] [CrossRef]
- Klammert, U.; Gbureck, U.; Vorndran, E.; Rödiger, J.; Meyer-Marcotty, P.; Kübler, A.C. 3D powder printed calcium phosphate implants for reconstruction of cranial and maxillofacial defects. J. Cranio-Maxillofacial Surg. 2010, 38, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.; Lindner, M.; Zhang, W.; Koczur, K.; Kirsten, A.; Telle, R.; Fischer, H. 3D printing of bone substitute implants using calcium phosphate and bioactive glasses. J. Eur. Ceram. Soc. 2010, 30, 2563–2567. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Chang, C.-C.; Ku, Y.C. New layer-based imaging and rapid prototyping techniques for computer-aided design and manufacture of custom dental restoration. J. Med. Eng. Technol. 2008, 32, 83–90. [Google Scholar] [CrossRef] [PubMed]
- O’reilly, M.K.; Reese, S.; Herlihy, T.; Geoghegan, T.; Cantwell, C.P.; Feeney, R.N.M.; Jones, J.F.X. Fabrication and Assessment of 3D Printed Anatomical Models of the Lower Limb for Anatomical Teaching and Femoral Vessel Access Training in Medicine. Anat. Sci. Educ. 2015. [CrossRef] [PubMed]
- Qiu, K.; Zhao, Z.; Haghiashtiani, G.; Guo, S.-Z.; He, M.; Su, R.; Zhu, Z.; Bhuiyan, D.B.; Murugan, P.; Meng, F.; et al. 3D Printed Organ Models with Physical Properties of Tissue and Integrated Sensors. Adv. Mater. Technol. 2017, 1700235, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Yang, Z.; Mongrain, R.; Leask, R.L.; Lachapelle, K. 3D printing materials and their use in medical education: a review of current technology and trends for the future. BMJ Simul. Technol. Enhanc. Learn. 2017. bmjstel-2017-000234. [Google Scholar] [CrossRef] [PubMed]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- ASTM F2792-10, Standard Terminology for Additive Manufacturing Technologies; ASTM International: West Conshohocken, PA, USA, 2010.
- Kim, G.B.; Lee, S.; Kim, H.; Yang, D.H.; Kim, Y.-H.; Kyung, Y.S.; Kim, C.-S.; Choi, S.H.; Kim, B.J.; Ha, H.; et al. Three-Dimensional Printing: Basic Principles and Applications in Medicine and Radiology. Korean J. Radiol. 2016, 17, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Boland, T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 2009, 30, 6221–6227. [Google Scholar] [CrossRef] [PubMed]
- Gaebel, R.; Ma, N.; Liu, J.; Guan, J.; Koch, L.; Klopsch, C.; Gruene, M.; Toelk, A.; Wang, W.; Mark, P.; et al. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials 2011, 32, 9218–9230. [Google Scholar] [CrossRef] [PubMed]
- Merceron, T.K.; Burt, M.; Seol, Y.J.; Kang, H.W.; Lee, S.J.; Yoo, J.J.; Atala, A. A 3D bioprinted complex structure for engineering the muscle-tendon unit. Biofabrication 2015, 7, 35003. [Google Scholar] [CrossRef] [PubMed]
- Palareti, G.; Legnani, C.; Cosmi, B.; Antonucci, E.; Erba, N.; Poli, D.; Testa, S.; Tosetto, A. Comparison between different D-Dimer cutoff values to assess the individual risk of recurrent venous thromboembolism: analysis of results obtained in the DULCIS study. Int. J. Lab. Hematol. 2016, 38, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.K.; Nieto, D.; Iglesias, L.; Goodarzi Hosseinabadi, H.; Maharjan, S.; Ruiz-Esparza, G.U.; Khoshakhlagh, P.; Manbachi, A.; Dokmeci, M.R.; Chen, S.; et al. Microfluidics-Enabled Multimaterial Maskless Stereolithographic Bioprinting. Adv. Mater. 2018, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oskui, S.M.; Diamante, G.; Liao, C.; Shi, W.; Gan, J.; Schlenk, D.; Grover, W.H. Assessing and Reducing the Toxicity of 3D-Printed Parts. Environ. Sci. Technol. Lett. 2016, 3, 1–6. [Google Scholar] [CrossRef]
- Xu, N.; Ye, X.; Wei, D.; Zhong, J.; Chen, Y.; Xu, G.; He, D. 3D artificial bones for bone repair prepared by computed tomography-guided fused deposition modeling for bone repair. ACS Appl. Mater. Interfaces 2014, 6, 14952–14963. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Alfredo, N.; Dorronsoro, A.; Cortajarena, A.L.; Rodríguez-Hernández, J. Antimicrobial 3D Porous Scaffolds Prepared by Additive Manufacturing and Breath Figures. ACS Appl. Mater. Interfaces 2017, 9, 37454–37462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melocchi, A.; Parietti, F.; Maroni, A.; Foppoli, A.; Gazzaniga, A.; Zema, L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int. J. Pharm. 2016, 509, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.; Hutmacher, D.W.; Castro, N.J. Independent evaluation of medical-grade bioresorbable filaments for fused deposition modelling/fused filament fabrication of tissue engineered constructs. Polymers (Basel). 2018, 10, 40. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Patrício, T.; Domingos, M.; Gloria, A.; Bártolo, P. Characterisation of PCL and PCL/PLA scaffolds for tissue engineering. Procedia CIRP 2013, 5, 110–114. [Google Scholar] [CrossRef]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Kucinska-Lipka, J.; Gubanska, I.; Strankowski, M.; Cieśliński, H.; Filipowicz, N.; Janik, H. Synthesis and characterization of cycloaliphatic hydrophilic polyurethanes, modified with L-ascorbic acid, as materials for soft tissue regeneration. Mater. Sci. Eng. C 2017, 75, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Cauich-rodríguez, J.V.; Chan-Chan, L.H.; Hernandez-Sánchez, F.; Cervantes-Uc, J.M. Degradation of Polyurethanes for Cardiovascular Applications. In Advances in Biomaterials Science and Biomedical Applications; Pignatello, R., Ed.; InTechOpen: Rijeka, Croatia, 2012; pp. 51–82. ISBN 978-953-51-1051-4. [Google Scholar]
- Davis, F.J.; Mitchell, G.R. Polyurethane Based Materials with Applications in Medical Devices. In Bio-Materials and Prototyping Applications in Medicine; CRC press: Washington, DC, USA, 2008; pp. 27–48. [Google Scholar]
- Kucińska-Lipka, J.; Gubanska, I.; Pokrywczynska, M.; Ciesliński, H.; Filipowicz, N.; Drewa, T.; Janik, H. Polyurethane porous scaffolds (PPS) for soft tissue regenerative medicine applications. Polym. Bull. 2017, 1–23. [Google Scholar] [CrossRef]
- Borkenhagen, M.; Stoll, R.C.; Neuenschwander, P.; Suter, U.W.; Aebischer, P. In vivo performance of a new biodegradable polyester urethane system used as a nerve guidance channel. Biomaterials 1998, 19, 2155–2165. [Google Scholar] [CrossRef]
- Lamba, N.M.K.; Woodhouse, K.A.; Cooper, S.L.; Lelah, M.D. Polyurethanes in biomedical applications; CRC press: Washington, DC, 1998; ISBN 9780849345173. [Google Scholar]
- Jung, S.Y.; Lee, S.J.; Kim, H.Y.; Park, H.S.; Wang, Z.; Kim, H.J.; Yoo, J.J.; Chung, S.M.; Kim, H.S. 3D printed polyurethane prosthesis for partial tracheal reconstruction: A pilot animal study. Biofabrication 2016, 8, 045015. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.J.; Dixon, S.; Hale, L.R.; Darbyshire, A.; Martin, D.; de Mel, A. Biomimetic heterogenous elastic tissue development. npj Regen. Med. 2017, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Kucińska-Lipka, J.; Gubanska, I.; Skwarska, A. Microporous Polyurethane Thin Layer as a Promising Scaffold for Tissue Engineering. Polymers (Basel). 2017, 9, 277. [Google Scholar] [CrossRef]
- Park, H.; Gong, M.-S.; Knowles, J.C. Catalyst-free synthesis of high elongation degradable polyurethanes containing varying ratios of isosorbide and polycaprolactone: physical properties and biocompatibility. J. Mater. Sci. Mater. Med. 2013, 24, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kang, M.-S.; Knowles, J.C.; Gong, M.-S. Synthesis of highly elastic biocompatible polyurethanes based on bio-based isosorbide and poly(tetramethylene glycol) and their properties. J. Biomater. Appl. 2014, 29, 454–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanzi, M.C.; Verderio, P.; Lampugnani, M.G.; Resnati, M.; Dejana, E.; Sturani, E. Cytotoxicity of some catalysts commonly used in the synthesis of copolymers for biomedical use. J. Mater. Sci. Mater. Med. 1994, 5, 393–396. [Google Scholar] [CrossRef]
- Hassan, M.; Mauritz, K.; Storey, R.; Wiggins, J. Biodegradable Aliphatic Thermoplastic Polyurethane Based on Poly(e-caprolactone) and L-Lysine Diisocyanate. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 2990–3000. [Google Scholar] [CrossRef]
- Heijkants, R.G.J.C.; Van Calck, R.V.; Van Tienen, T.G.; De Groot, J.H.; Buma, P.; Pennings, A.J.; Veth, R.P.H.; Schouten, A.J. Uncatalyzed synthesis, thermal and mechanical properties of polyurethanes based on poly(ε-caprolactone) and 1,4-butane diisocyanate with uniform hard segment. Biomaterials 2005, 26, 4219–4228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrioni, B.R.; De Carvalho, S.M.; Oréfice, R.L.; De Oliveira, A.A.R.; Pereira, M.D.M. Synthesis and characterization of biodegradable polyurethane films based on HDI with hydrolyzable crosslinked bonds and a homogeneous structure for biomedical applications. Mater. Sci. Eng. C 2015, 52, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Janik, H.Z. Struktury nadcząsteczkowe i wybrane właściwości rozgałęzionych i usieciowanych poli(estro-uretanów), poli(etero-uretanów) i poli(uretano-biuretów) formowanych reaktywnie. Zesz. Nauk. Politech. Gdańskiej. Chem. 2005, Nr 53(599), 3–141. [Google Scholar]
- Pietrzak, K.; Isreb, A.; Alhnan, M.A. A flexible-dose dispenser for immediate and extended release 3D printed tablets. Eur. J. Pharm. Biopharm. 2015, 96, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Rizvi, G.M.; Bellehumeur, C.T.; Gu, P. Effect of processing conditions on the bonding quality of FDM polymer filaments. Rapid Prototyp. J. 2008, 14, 72–80. [Google Scholar] [CrossRef]
- Gkartzou, E.; Koumoulos, E.P.; Charitidis, C.A. Production and 3D printing processing of bio-based thermoplastic filament. Manuf. Rev. 2017, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Nezarati, R.M.; Eifert, M.B.; Dempsey, D.K.; Cosgriff-Hernandez, E. Electrospun vascular grafts with improved compliance matching to native vessels. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, R.; Zhao, Y.; Hu, Z.; Li, X. Preparation of Dipyridamole/Polyurethane Core–Shell Nanofibers by Coaxial Electrospinning for Controlled-Release Antiplatelet Application. J. Nanosci. Nanotechnol. 2016, 16, 6860–6866. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Y.; Fang, Z.; Yuan, W.; Khan, M. Co-electrospun blends of PU and PEG as potential biocompatible scaffolds for small-diameter vascular tissue engineering. Mater. Sci. Eng. C 2012, 32, 2306–2315. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Y.; An, B.; Zhang, W.; Sun, M.; Fang, Z.; Yuan, W.; Khan, M. Fabrication of PU/PEGMA crosslinked hybrid scaffolds by in situ UV photopolymerization favoring human endothelial cells growth for vascular tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Feng, Y.; Wang, H.; Yang, D.; An, B.; Zhang, W.; Khan, M.; Guo, J. Hemocompatible surface of electrospun nanofibrous scaffolds by ATRP modification. Mater. Sci. Eng. C 2013, 33, 3644–3651. [Google Scholar] [CrossRef] [PubMed]
- NinjaTek Technical Sepcification of NinjaFlex 3D Printing Filament. Available online: https://ninjatek.com/wp-content/uploads/2016/05/NinjaFlex-TDS.pdf (accessed on 4 March 2018).
- Chen, R.; Huang, C.; Ke, Q.; He, C.; Wang, H.; Mo, X. Preparation and characterization of coaxial electrospun thermoplastic polyurethane/collagen compound nanofibers for tissue engineering applications. Colloids Surfaces B Biointerfaces 2010, 79, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Detta, N.; Errico, C.; Dinucci, D.; Puppi, D.; Clarke, D.A.; Reilly, G.C.; Chiellini, F. Novel electrospun polyurethane/gelatin composite meshes for vascular grafts. J. Mater. Sci. Mater. Med. 2010, 21, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Filoalfa–Bioflex Filament. Available online: https://www.filoalfa3d.com/en/filaments-175mm/296-bioflex-pla-shore-27d-white-o-175-mm-8050327032385.html (accessed on 4 March 2018).
- Kucinska-Lipka, J.; Gubanska, I.; Sienkiewicz, M. Thermal and mechanical properties of polyurethanes modified with L-ascorbic acid. J. Therm. Anal. Calorim. 2017, 127, 1631–1638. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; third; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; ISBN 978-0-470-09307-8. [Google Scholar]
- Yilgor, I.; Yilgor, E.; Guler, I.G.; Ward, T.C.; Wilkes, G.L. FTIR investigation of the influence of diisocyanate symmetry on the morphology development in model segmented polyurethanes. Polymer (Guildf). 2006, 47, 4105–4114. [Google Scholar] [CrossRef]
- Janik, H. Progress in the studies of the supermolecular structure of segmented polyurethanes. Polimery 2010, 55, 419–500. [Google Scholar]
- Menzies, K.L.; Jones, L. The impact of contact angle on the biocompatibility of biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Rodrigues, A.; Chang, D. Ferogin Body Reaction To Biometerials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Fromstein, J.D.; Woodhouse, K.A. Elastomeric biodegradable polyurethane blends for soft tissue applications. J. Biomater. Sci. Polym. Ed. 2002, 13, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Du, B.; Wang, Y.; Qian, S.; Li, W.; Gao, Y.; Sun, M.; Luan, S.; Yin, J. Hemocompatibility Evaluation of Polyurethane Film with Surface-Grafted Sugar- Based Amphipathic Compounds. J. Anal. Bioanal. Tech. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Tahara, D.; Oikawa, N.; Kurita, R. Mobility enhancement of red blood cells with biopolymers. J. Phys. Soc. Japan 2016, 85, 10–12. [Google Scholar] [CrossRef]
- Keshel, S.H.; Azhdadi, S.N.K.; Asefnejad, A.; Sadraeian, M.; Montazeri, M.; Biazar, E. The relationship between cellular adhesion and surface roughness for polyurethane modified by microwave plasma radiation. Int. J. Nanomedicine 2011, 6, 641–647. [Google Scholar] [CrossRef] [PubMed]









| Compound | Supplier | Description | Structure Formula |
|---|---|---|---|
| BDO | Brenntag, Germany | Low molecular chain extender, Mol mass = 88 g/mol, Physical state–clear liquid, Purity > 95.5%, Tm = 204 °C, Boiling point ~ 230 °C, ρ (20 °C) = 1020 g/cm3 |  |
| HDI | Sigma-Aldrich, Germany | Aliphatic diisocyanate, colorless liquid. Boiling point = 255 °C, Flash point = 130 °C, ρ (25 °C) = 1.05 g/cm3, Purity > 99%, Tm = −67 °C, Soluble in water, LD50 (rat) = 746 mg/kg. |  |
| PEBA (POLIOS 55/20) | Purinova, Poland | Ester-based polyol, Mol mass = 2000 g/mol, Hydroxyl number = 54–58, Acid number–max. 0.6. |  |
| Lp. | Zones Temperature Profile [°C] | Operating Parameters | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | Coupler | Head | Rotation speed [rpm] | Head pressure [bar] | Load [%] | |
| 1 | 160 | 165 | 170 | 175 | 185 | 185 | 190 | 195 | 190 | 190 | 185 | 20 | 37–48 | 45–50 |
| 2 | 170 | 175 | 175 | 180 | 190 | 200 | 205 | 200 | 200 | 195 | 195 | 20 | 28–30 | 20–28 |
| 3 * | 170 | 175 | 180 | 190 | 200 | 205 | 210 | 210 | 205 | 200 | 200 | 20 | 17–18 | 15–18 |
| 4 | 170 | 175 | 180 | 190 | 195 | 205 | 210 | 213 | 217 | 215 | 210 | 20 | 3–6 | 5–7 |
| S-TPU | F-TPU | Band | Description |
|---|---|---|---|
| Wavelength (cm−1) | |||
| 3324 w | 3324 w | νNH | Stretching of NH groups. These groups were hydrogen bonded with C=O of ester groups present in macrodiol. |
| 2941 w, 2863 w | 2939 w, 2865 w | νCH2, νCH3 | Stretching of aliphatic asymmetric and symmetric CH2 groups present in the S-TPU chain and in the S-TPU filament |
| 1730 vs. −1686 s | 1733 vs. −1685 s | νC=O | stretching of C=O in ester groups of macrodiol,(hydrogen bonded and not hydrogen bonded) |
| 1535 s | 1535 s | νC–N | Stretching of C–N in urethane group |
| 1459 w–1336 vw | 1465 w–1346 w | δCH2 | deformation vibrations of aliphatic CH2 groups present in the S-TPU and S-TPU filament: bending, wagging, scissoring in plane |
| 1259 m–1219 m | 1257 s–1216 m | νC–(C=O)–O | Stretching vibrations of –C–(C=O)–O– (ester group), not hydrogen bonded |
| 1165 s | 1165 m | νNH–(C=O)–O | Stretching vibrations of –NH–(C=O)–O– of urethane group |
| 1129 s–994 w | 1135 s–947 m | νC–(C=O)–O νC–O | Stretching vibration of hydrogen bonded –C–(C=O)–O–, |
| 873 w–642 w | 873 w–638 m | δCH2, δNH, δOH | out of the plane deformation of CH2(scissoring/wagging) as well as NH and OH groups (scissoring and wagging). |
| Value Range | MilaMed® | Desmopan® AU | Texin®RxT50 | S-TPU |
|---|---|---|---|---|
| TSb [MPa] | 15–30 | 25–50 | 25–52 | 26 |
| Eb [%] | 540–565 | 470–880 | 320–770 | 705 |
| HS [°Sh A/D] | no data found | 60A–75D | 70A–65D | 26D |
| Chemical composition | Aliphatic polyether | Aromatic polyester | Aromatic polyether | Aliphatic polyester |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haryńska, A.; Gubanska, I.; Kucinska-Lipka, J.; Janik, H. Fabrication and Characterization of Flexible Medical-Grade TPU Filament for Fused Deposition Modeling 3DP Technology. Polymers 2018, 10, 1304. https://doi.org/10.3390/polym10121304
Haryńska A, Gubanska I, Kucinska-Lipka J, Janik H. Fabrication and Characterization of Flexible Medical-Grade TPU Filament for Fused Deposition Modeling 3DP Technology. Polymers. 2018; 10(12):1304. https://doi.org/10.3390/polym10121304
Chicago/Turabian StyleHaryńska, Agnieszka, Iga Gubanska, Justyna Kucinska-Lipka, and Helena Janik. 2018. "Fabrication and Characterization of Flexible Medical-Grade TPU Filament for Fused Deposition Modeling 3DP Technology" Polymers 10, no. 12: 1304. https://doi.org/10.3390/polym10121304