Facile Fabrication of Magnetic, Durable and Superhydrophobic Cotton for Efficient Oil/Water Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
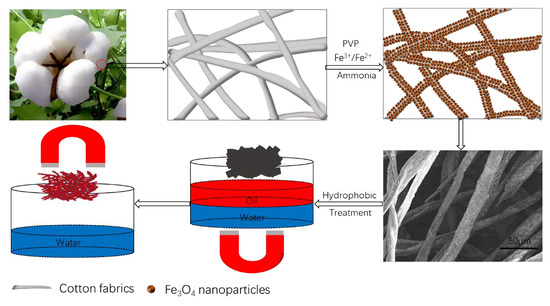
2.2. Preparation of PVP-Modified Cotton
2.3. Preparation of Fe3O4-Modified Cotton
2.4. Fabrication of Superhydrophobic Cotton
2.5. Characterization
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ramirez, M.I.; Arevalo, A.P.; Sotomayor, S.; Bailon-Moscoso, N. Contamination by oil crude extraction e Refinement and their effects on human health. Environ. Pollut. 2015, 231, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Corina, P.D.B.; Louis, P.; Siham, B.; Lukas, Y.W.; Birgit, W.; Jan, W.; Arey, J.S.; Bart, V.D.B.; Arjen, J.; Johannes, H.; et al. Immediate ecotoxicological effects of short-lived oil spills on marine biota. Nat. Commun. 2016, 7, 11206–11213. [Google Scholar]
- Chen, W.B.; He, H.; Zhu, H.X.; Cheng, M.X.; Li, Y.H.; Wang, S.F. Thermo-responsive cellulose-based material with switchable wettability for controllable oil/water separation. Polymers 2018, 10, 592. [Google Scholar] [CrossRef]
- Kwon, G.; Kota, A.K.; Li, Y.; Sohani, A.; Mabry, J.M.; Tuteja, A. On-demand separation of oil-water mixtures. Adv. Mater. 2012, 24, 3666–3671. [Google Scholar] [CrossRef] [PubMed]
- Naseem, S.; Wu, C.M.; Xu, T.Z.; Lai, C.C.; Rwei, S.P. Oil-water separation of electrospun cellulose triacetate nanofiber membranes modified by electrophoretically deposited TiO2/graphene oxide. Polymers 2018, 10, 746. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Z.; Mai, Z.; Ma, Y.; Liu, B.; Jiang, L.; Zhu, D. A super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water. Angew. Chem. Int. Ed. 2004, 43, 2012–2014. [Google Scholar] [CrossRef] [PubMed]
- Abdul, R.S.; Rita, M.; Kantesh, B. Superhydrophobic self-floating carbon nanofiber coating for efficient gravity-directed oil/water separation. J. Mater. Chem. A 2017, 5, 2936–2946. [Google Scholar]
- Li, J.; Xu, C.; Guo, C.; Tian, H.; Zha, F.; Guo, L. Underoil superhydrophilic desert sand layer for efficient gravity-directed water-in-oil emulsions separation with high flux. J. Mater. Chem. A 2018, 6, 223–230. [Google Scholar] [CrossRef]
- Muhammad, Z.K.; Vijay, B.; Jiri, M.; Azam, A.; Martina, V. Superhydrophobicity, UV protection and oil/water separation properties of fly ash/Trimethoxy(octadecyl)silane coated cotton fabrics. Carbohydr. Polym. 2018, 202, 571–580. [Google Scholar]
- Bu, X.; Lu, Y.; Chen, S.; Li, D.; Zhang, Z.; Qian, P. Fabrication of porous carbon nitride foams/acrylic resin composites for efficient oil and organic solvents capture. Chem. Eng. J. 2019, 355, 299–308. [Google Scholar] [CrossRef]
- Paul, U.C.; Fragouli, D.; Bayer, I.S.; Athanassiou, A. Functionalized cellulose networks for efficient oil removal from oil–water emulsions. Polymers 2016, 8, 52. [Google Scholar] [CrossRef]
- Yin, K.; Yang, S.; Dong, X.R.; Chu, D.K.; Duan, J.A.; He, J. Robust laser-structured asymmetrical PTFE mesh for underwater directional transportation and continuous collection of gas bubbles. Appl. Phys. Lett. 2018, 112, 243701–243705. [Google Scholar] [CrossRef]
- Liu, H.; Kang, Y. Superhydrophobic and superoleophilic modified EPDM foam rubber fabricated by a facile approach for oil/water separation. Appl. Surf. Sci. 2018, 451, 223–231. [Google Scholar] [CrossRef]
- Li, J.T.; Tenjimbayashi, M.; Zacharia, N.S.; Shiratori, S. One-Step Dipping Fabrication of Fe3O4/PVDF-HFP Composite 3D Porous Sponge for Magnetically Controllable Oil–Water Separation. ACS Sustain. Chem. Eng. 2018, 6, 10706–10713. [Google Scholar] [CrossRef]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Dunderdale, G.J.; Urata, C.; Sato, T.; England, M.W.; Hozumi, A. Continuous, High-Speed, and Efficient Oil/Water Separation using Meshes with Antagonistic Wetting Properties. ACS Appl. Mater. Interfaces 2015, 7, 18915–18919. [Google Scholar] [CrossRef] [PubMed]
- Dunderdale, G.J.; England, M.W.; Sato, T.; Urata, C.; Hozumi, A. Programmable Oil/Water Separation Meshes: Water or Oil Selectivity Using Contact Angle Hysteresis. Macromol. Mater. Eng. 2016, 301, 1032–1036. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Yang, J.H.; Li, L.L.; Cui, C.X.; Li, Y.; Liu, S.Q.; Zhou, X.M.; Qu, L.B. Facile fabrication of superhydrophobic copper- foam and electrospinning polystyrene fiber for combinational oil–water separation. Polymers 2019, 11, 97. [Google Scholar] [CrossRef]
- Ma, Q.; Li, G.D.; Liu, X.Y.; Wang, Z.; Song, Z.; Wang, H.T. Zeolitic imidazolate framework-8 film coated stainless steel meshes for highly efficient oil/water separation. Chem. Commun. 2018, 54, 5530–5533. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, M.G.; Chen, G.X.; Chen, Q.F.; Tian, J.F. Robust fabrication of fluorine-free superhydrophobic steel mesh for efficient oil/water separation. J. Mater. Sci. 2017, 52, 2549–2559. [Google Scholar] [CrossRef]
- Yu, M.G.; Wang, Q.; Zhang, M.; Deng, Q.J.; Chen, D.C. Facile fabrication of raspberry-like composite microspheres for the construction of superhydrophobic films and applications in highly efficient oil–water separation. RSC Adv. 2017, 7, 39471–39479. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Wu, L.H.; Lu, S.C.; Lin, X.X.; Xiao, H.; Ouyang, X.H.; Cao, S.L.; Chen, L.H.; Huang, L.L. Robust superhydrophobic and superoleophilic filter paper via atom transfer radical polymerization for oil/water separation. Carbohydr. Polym. 2018, 181, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Camila, F.M.; Jeferson, A.V.; Marianny, Y.C.; Cristian, B. Separation of asphaltene-stabilized water in oil emulsions and immiscible oil/water mixtures using a hydrophobic cellulosic membrane. Fuel 2018, 231, 297–306. [Google Scholar]
- Roy, S.; Zhai, L.D.; Hai, L.V.; Kim, J.W.; Park, J.H.; Kim, H.C.; Kim, J. One-step nanocellulose coating converts tissue paper into an efficient separation membrane. Cellulose 2018, 25, 4871–4886. [Google Scholar] [CrossRef]
- Bai, W.B.; Guan, M.Q.; Lai, N.S.; Yao, R.J.; Xu, Y.L.; Lin, J.H. Superhydrophobic paper from conjugated poly(p-phenylene)s: Self-assembly and separation of oil/water mixture. Mater. Chem. Phys. 2018, 216, 230–236. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Liu, K.; Jiang, L. Bioinspired Multifunctional Foam with Self-Cleaning and Oil/Water Separation. Adv. Funct. Mater. 2013, 23, 2881–2886. [Google Scholar] [CrossRef]
- Lv, X.S.; Tian, D.H.; Peng, Y.Y.; Li, J.X.; Jiang, G.M. Superhydrophobic magnetic reduced graphene oxide-decorated foam for efficient and repeatable oil-water separation. Appl. Surf. Sci. 2019, 466, 937–945. [Google Scholar] [CrossRef]
- Li, L.; Liu, L.; Lei, J.; He, J.X.; Li, N.B.; Pan, F.S. Intelligent sponge with reversibly tunable super-wettability: Robust for effective oil-water separation as both the absorber and filter tolerate fouling and harsh environments. J. Mater. Chem. A 2016, 4, 12334–12340. [Google Scholar] [CrossRef]
- Qiang, F.; Hu, L.; Gong, L.; Zhao, L.; Li, S.; Tang, L. Facile synthesis of super-hydrophobic, electrically conductive and mechanically flexible functionalized graphene nanoribbon/polyurethane sponge for efficient oil/water separation at static and dynamic states. Chem. Eng. J. 2018, 334, 2154–2166. [Google Scholar] [CrossRef]
- Li, Y.B.; Feng, Z.L.; He, Y.; Fan, Y.; Ma, J.; Yin, X.Y. Facile way in fabricating a cotton fabric membrane for switchable oil/water separation and water purification. Appl. Surf. Sci. 2018, 441, 500–507. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, Z.; Yang, H.; Hou, K.; Zeng, X.; Zheng, Y.; Cheng, J. Nature-Inspired Strategy toward Superhydrophobic Fabrics for Versatile Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 9184–9194. [Google Scholar] [CrossRef] [PubMed]
- Anita, P.; Priya, V.; Soumya, S.M.; Aditya, K. Development of liquid repellent coating on cotton fabric by simple binary silanization with excellent self-cleaning and oil-water separation properties. Carbohydr. Polym. 2018, 181, 1052–1060. [Google Scholar]
- Cheng, Q.; Guan, C.; Wang, M.; Li, Y.; Zeng, J. Cellulose nanocrystal coated cotton fabric with superhydrophobicity for efficient oil/water separation. Carbohydr. Polym. 2018, 199, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.G.; Lin, B.B.; Chen, S.X.; Deng, Q.J.; Liu, G.; Wang, Q. Biomimetic fabrication of superhydrophobic loofah sponge: Robust for highly efficient oil–water separation in harsh environments. RSC Adv. 2018, 8, 24297–24304. [Google Scholar] [CrossRef]
- Yan, T.; Chen, X.Q.; Zhang, T.H.; Yu, J.G.; Jiang, X.Y.; Hu, W.J.H.; Jiao, F.P. A magnetic pH-induced textile fabric with switchable wettability for intelligent oil/water separation. Chem. Eng. J. 2018, 347, 52–63. [Google Scholar] [CrossRef]
- Huang, Y.W.; Shan, Y.X.; Liang, S.; Zhao, X.L.; Jiang, G.; Hu, C.Y.; Yang, J.X.; Liu, L.L. Coordinated silicon elastomer coating@fabrics with oil/water separation capabilities, outstanding durability and ultra-fast room-temperature self-healing ability. J. Mater. Chem. A 2018, 6, 17156–17163. [Google Scholar] [CrossRef]
- Mulyadi, A.; Zhang, Z.; Deng, Y. Fluorine-Free Oil Absorbents Made from Cellulose Nanofibril Aerogels. ACS Appl. Mater. Interfaces 2016, 8, 2732–2740. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhu, P.; Yu, S.; Sun, R.C.; Wong, C.; Liao, W. Ultralight, super-elastic and volume-preserving cellulose fiber/graphene aerogel for high-performance electromagnetic interference shielding. Carbon 2017, 115, 629–639. [Google Scholar] [CrossRef]
- Li, Z.; Shao, L.; Hu, W.; Zheng, T.; Lu, L.; Cao, Y.; Chen, Y. Excellent reusable chitosan/cellulose aerogel as an oil and organic solvent absorbent. Carbohydr. Polym. 2018, 191, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, M.; Zang, D.; Gao, Z.; Wang, C. Fabrication of superhydrophobic/superoleophilic cotton for application in the field of water/oil separation. Carbohydr. Polym. 2014, 103, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Geng, G.; Wang, A.; Liu, X.; Du, J.; Zou, Z.; Zhang, S.; Han, F. Double biomimetic fabrication of robustly superhydrophobic cotton fiber and its application in oil spill cleanup. Ind. Crops Prod. 2015, 77, 36–43. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, M.G.; Chen, G.X.; Chen, Q.F.; Tai, J.L. Facile Fabrication of Superhydrophobic/Superoleophilic Cotton for Highly Efficient Oil/Water Separation. Bioresources 2017, 12, 643–654. [Google Scholar] [CrossRef]
- Farshad, B.; Hossein, K.; Masoud, S. Recyclable magnetic superhydrophobic straw soot sponge for highly efficient oil/water separation. J. Colloid Interface Sci. 2017, 497, 57–65. [Google Scholar]
- Liu, L.; Lei, J.; Li, L.; Zhang, R.; Mi, N.; Chen, H.; Huang, D.; Li, N. A facile method to fabricate the superhydrophobic magnetic sponge for oil-water separation. Mater. Lett. 2017, 195, 66–70. [Google Scholar] [CrossRef]
- Yu, L.; Yang, H.; Wang, Y.; Jiang, W. Magnetically enhanced superhydrophobic functionalized polystyrene foam for the high efficient cleaning of oil spillage. Powder Technol. 2017, 311, 257–264. [Google Scholar] [CrossRef]
- Yu, L.; Hao, G.; Xiao, L.; Yin, Q.; Xia, M.; Jiang, W. Robust magnetic polystyrene foam for high efficiency and removal oil from water surface. Sep. Purif. Technol. 2017, 173, 121–128. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Yang, X.B.; Wang, Z.X.; Long, J.; Shao, L. Designing multifunctional 3D magnetic foam for effective insoluble oil separation and rapid selective dye removal for use in wastewater remediation. J. Mater. Chem. A 2017, 5, 7316–7325. [Google Scholar] [CrossRef]
- Fang, J.; Wang, H.; Xue, Y.; Wang, X.; Lin, T. Magnet-Induced Temporary Superhydrophobic Coatings from One-Pot Synthesized Hydrophobic Magnetic Nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, G.X.; Yu, Z.H.; Ouyang, X.P.; Tian, J.F.; Yu, M.G. Photoluminescent composites of lanthanide-based nanocrystal-functionalized cellulose fibers for anticounterfeiting applications. ACS Sustain. Chem. Eng. 2018, 6, 13960–13967. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, Y.; Zhang, G.; Zhang, F. An eco-friendly intumescent flame retardant with high efficiency and durability for cotton fabric. Cellulose 2018, 25, 5389–5404. [Google Scholar] [CrossRef]
- Weng, X.; Ma, L.; Guo, M.; Su, Y.; Dharmarajan, R.; Chen, Z. Removal of doxorubicin hydrochloride using Fe3O4 nanoparticles synthesized by euphorbia cochinchinensis extract. Chem. Eng. J. 2018, 353, 482–489. [Google Scholar] [CrossRef]
- Zhang, Z.; Chang, H.; Xue, B.; Zhang, S.; Li, X.; Wong, W.; Li, K.; Zhu, X. Near-infrared and visible dual emissive transparent nanopaper based on Yb(III)-carbon quantum dots grafted oxidized nanofibrillated cellulose for anti-counterfeiting applications. Cellulose 2018, 25, 377–389. [Google Scholar] [CrossRef]
- Monmi, G.; Archana, M.D. Synthesis of cellulose impregnated copper nanoparticles as an efficient heterogeneous catalyst for CN coupling reactions under mild conditions. Carbohydr. Polym. 2018, 195, 189–198. [Google Scholar]
- Ye, J.; Wang, B.; Xiong, J.; Sun, R.C. Enhanced fluorescence and structural characteristics of carboxymethyl cellulose/Eu(III) nano-complex: Influence of reaction time. Carbohydr. Polym. 2016, 135, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.T.; Lin, B.; Jiang, L.W.; Lin, E.C.; Chen, J.; Zhang, S.J.; Tang, Y.W.; He, F.A.; Li, D.H. Effective preparation of magnetic superhydrophobic Fe3O4/PU sponge for oil-water separation. Appl. Surf. Sci. 2018, 427, 56–64. [Google Scholar] [CrossRef]
- Mi, H.Y.; Jing, X.; Xie, H.; Huang, H.X.; Turng, L.S. Magnetically driven superhydrophobic silica sponge decorated with hierarchical cobalt nanoparticles for selective oil absorption and oil/water separation. Chem. Eng. J. 2018, 337, 541–551. [Google Scholar] [CrossRef]
- Hayaka, F.; Tsuguyuki, S.; Tadahisa, I.; Yoshiaki, K.; Akira, I. Transparent and High Gas Barrier Films of Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation. Biomacromolecules 2009, 10, 162–165. [Google Scholar]
- Xiao, S.B.; Zhang, Z.L.; He, J.Y. Atomistic dewetting mechanics of Wenzel and monostable Cassie–Baxter states. Phys. Chem. Chem. Phys. 2018, 20, 24759–24767. [Google Scholar] [CrossRef] [PubMed]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Wang, Q.; Yang, W.; Xu, Y.; Zhang, M.; Deng, Q.; Liu, G. Facile Fabrication of Magnetic, Durable and Superhydrophobic Cotton for Efficient Oil/Water Separation. Polymers 2019, 11, 442. https://doi.org/10.3390/polym11030442
Yu M, Wang Q, Yang W, Xu Y, Zhang M, Deng Q, Liu G. Facile Fabrication of Magnetic, Durable and Superhydrophobic Cotton for Efficient Oil/Water Separation. Polymers. 2019; 11(3):442. https://doi.org/10.3390/polym11030442
Chicago/Turabian StyleYu, Mingguang, Qing Wang, Wenxin Yang, Yonghang Xu, Min Zhang, Qianjun Deng, and Guang Liu. 2019. "Facile Fabrication of Magnetic, Durable and Superhydrophobic Cotton for Efficient Oil/Water Separation" Polymers 11, no. 3: 442. https://doi.org/10.3390/polym11030442