Plasticization Effect of Bio-Based Plasticizers from Soybean Oil for Tire Tread Rubber
Abstract
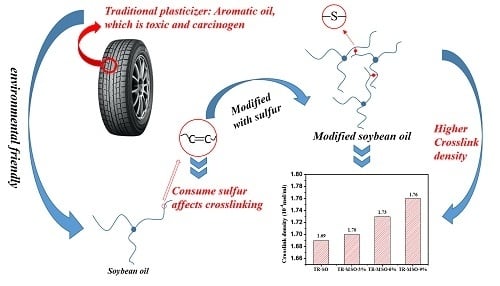
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Modified Soybean Oil (MSO)
2.3. Preparation of Rubber Compounds and Rubber Composites
2.4. Methods
3. Results
3.1. Synthesis and Characterization of Soybean-Oil-Based Plasticizer
3.2. Plasticization Effect of Soybean-Oil-Based Plasticizers with Different Sulfur
3.2.1. Processing Property of the Rubber Compounds
3.2.2. Curing Behavior
3.2.3. Mechanical Property
3.3. Plasticization Effect with Different Quantities of MSO-6%
3.3.1. Processing Properties
3.3.2. Curing Characteristics
3.3.3. Mechanical Properties of Tread Rubber with Different Quantity of Plasticizer
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wypych, G. Handbook of Plasticizers; ChemTec Publishing: Toronto, ON, Canada, 2004. [Google Scholar]
- Bodaghi, A. An overview on the recent developments in reactive plasticizers in polymers. Polym. Adv. Technol. 2019, 31, 355–367. [Google Scholar] [CrossRef]
- Dasgupta, S.; Agrawal, S.; Bandyopadhyay, S.; Chakraborty, S.; Mukhopadhyay, R.; Malkani, R.; Ameta, S. Characterization of eco-friendly processing aids for rubber compound. Polym. Test. 2007, 26, 489–500. [Google Scholar] [CrossRef]
- Parks, L.G. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol. Sci. 2000, 58, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Delpech, M.C.; Mello, I.L.; Delgado, F.C.S.; Sousa, J.M. Evaluation of thermal and mechanical properties of rubber compositions based on SBR extended with safe oils. J. Appl. Polym. Sci. 2012, 125, 4074–4081. [Google Scholar] [CrossRef]
- Dasgupta, S.; Agrawal, S.L.; Bandyopadhyay, S.; Mukhopadhyay, R.; Malkani, R.K.; Ameta, S.C. Eco-friendly processing oils: A new tool to achieve the improved mileage in tyre tread. Polym. Test. 2009, 28, 251–263. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, Y.H.; Zhang, L.Q.; Zhao, Y.; Vyzhimov, R.; Tan, T.W.; Fong, H. Investigation of Palm Oil as Green Plasticizer on the Processing and Mechanical Properties of Ethylene Propylene Diene Monomer Rubber. Ind. Eng. Chem. Res. 2016, 55, 2784–2789. [Google Scholar] [CrossRef]
- Bocque, M.; Voirin, C.; Lapinte, V.; Caillol, S.; Robin, J.J. Petro-Based and Bio-Based Plasticizers: Chemical Structures to Plasticizing Properties. J. Polym. Sci. A 2016, 54, 11–33. [Google Scholar] [CrossRef]
- Alexander, M.; Thachil, E.T. A comparative study of cardanol and aromatic oil as plasticizers for carbon-black-filled natural rubber. J. Appl. Polym. Sci. 2006, 102, 4835–4841. [Google Scholar] [CrossRef]
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, T.R.; Chauhan, R.C.; Choe, S.; Kundu, P.P. Effect of the doses and nature of vegetable oil on carbon black/rubber interactions: Studies on castor oil and other vegetable oils. J. Appl. Polym. Sci. 2003, 87, 1574–1578. [Google Scholar] [CrossRef]
- Bueno-Ferrer, C.; Garrigos, M.C.; Jimenez, A. Characterization and thermal stability of poly(vinyl chloride) plasticized with epoxidized soybean oil for food packaging. Polym. Degrad. Stab. 2010, 95, 2207–2212. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Wang, R.G.; King, H.L.; Qiao, B.; Ma, J.; Zhang, L.Q.; Wang, H. Synthesis and Characterization of Novel Soybean-Oil-Based Elastomers with Favorable Processability and Tunable Properties. Macromolecules 2012, 45, 9010–9019. [Google Scholar] [CrossRef]
- Wang, Z.; Han, Y.; Zhang, X.; Huang, Z.H.; Zhang, L.Q. Plasticization Effect of Transgenic Soybean Oil. I. On Ethylene Propylene Diene Monomer (EPDM), as Substitute for Paraffin Oil. J. Appl. Polym. Sci. 2013, 130, 4457–4463. [Google Scholar] [CrossRef]
- Li, J.X.; Isayev, A.I.; Ren, X.F.; Soucek, M.D. Modified soybean oil-extended SBR compounds and vulcanizates filled with carbon black. Polymer 2015, 60, 144–156. [Google Scholar] [CrossRef]
- Qin, X.; He, Y.; Khan, S.; Zhang, B.H.; Chen, F.X.; Dong, D.; Wang, Z.; Zhang, L.Q. Controllable Synthesis and Characterization of Soybean-Oil-Based Hyperbranched Polymers via One-Pot Method. ACS Sustainable Chem. Eng. 2018, 6, 12865–12871. [Google Scholar] [CrossRef]
- Wang, Z.; Han, Y.; Huang, Z.H.; Zhang, X.; Zhang, L.Q.; Lu, Y.L.; Tan, T.W. Plasticization Effect of Hydrogenated Transgenic Soybean Oil on Nitrile-Butadiene Rubber. J. Appl. Polym. Sci. 2014, 131, 40643. [Google Scholar] [CrossRef]
- Fankhauser-Noti, A.; Fiselier, K.; Biedermann, S.; Biedermann, M.; Grob, K.; Armellini, F.; Rieger, K.; Skjevrak, I. Epoxidized soy bean oil (ESBO) migrating from the gaskets of lids into food packed in glass jars. Eur. Food Res. Technol. 2005, 221, 416–422. [Google Scholar] [CrossRef]
- Castle, L.; Mayo, A.; Gilbert, J. Migration of epoxidised soya bean oil into foods from retail packaging materials and from plasticised PVC film used in the home. Food Addit. Contam. 1990, 7, 29–36. [Google Scholar] [CrossRef]
- Ayunie, N.; Zaki, M.; Romli, A.; Wahab, M.; Abidin, M. Effect of Epoxidized Palm Oils Loading on the Crosslink Density and Rebound Resilience Characteristic of SBR Vulcanizates. Proc. Adv. Mater. Res. 2013, 748, 206–210. [Google Scholar] [CrossRef]
- Novakov, I.A.; Nistratov, A.V.; Vaniev, M.A.; Luk’Yanichev, V.V.; Zershchikov, K.Y. Effect of plasticizer nature on the cure rheokinetics and structure of Thiokol sealant vulcanizates. Polym. Sci. 2007, 49, 71–73. [Google Scholar] [CrossRef]







| Ingredient | Recipe 1 (phr) | Recipe 2 (phr) |
|---|---|---|
| SBR | 20.0 | 20.0 |
| NR | 40.0 | 40.0 |
| BR | 40.0 | 40.0 |
| Plasticizer | 0, 25 Variable (SO, MSO-3S%, MSO-6%, MSO-9%) | MSO-6% Variable(5,10,15,20,25), AO(25) |
| Silica | 35.0 | 35.0 |
| Carbon black | 40.0 | 40.0 |
| TESPT | 2.8 | 2.8 |
| SA | 2.0 | 2.0 |
| Zinc oxide | 2.5 | 2.5 |
| Antioxidant 4020 | 1.5 | 1.5 |
| Accelerator CZ | 1.7 | 1.7 |
| Sulfur | 1.9 | 1.9 |
| Sample | Mn (×103 g/mol) | Mw (×103 g/mol) | Area Percentage (%) |
|---|---|---|---|
| SO | 1.07 | 1.24 | 100 |
| MSO-3% | 3.30 | 3.63 | 17.72 |
| 1.10 | 1.30 | 82.28 | |
| MSO-6% | 3.90 | 4.98 | 42.33 |
| 1.12 | 1.33 | 57.67 | |
| MSO-9% | 4.69 | 7.18 | 57.10 |
| 1.12 | 1.34 | 42.90 |
| Number | MH (dNm) | ML (dNm) | ΔM (dNm) | T90 (min:sec) | Crosslink Density (10−4mol/mL) |
|---|---|---|---|---|---|
| Reference sample | 52.33 | 14.28 | 38.05 | 8:29 | / |
| TR-SO | 27.01 | 7.96 | 19.05 | 7:31 | 1.69 |
| TR-MSO-3% | 27.14 | 7.98 | 19.16 | 5:28 | 1.70 |
| TR-MSO-6% | 29.41 | 8.91 | 20.50 | 4:31 | 1.73 |
| TR-MSO-9% | 31.24 | 10.18 | 21.06 | 4:36 | 1.76 |
| Sample | Elongation at Break (%) | Modulus at 100% (MPa) | Modulus at 300% (MPa) | Tensile Stress (MPa) | Shore A Hardness |
|---|---|---|---|---|---|
| Reference sample | 328 ± 17 | 3.3 ± 0.1 | 16.6 ± 0.2 | 17.2 ± 1.7 | 73 |
| TR-SO | 618 ± 31 | 1.6 ± 0.1 | 7.7 ± 0.1 | 20.0 ± 1.4 | 55 |
| TR-MSO-3% | 553 ± 27 | 2.0 ± 0.3 | 8.8 ± 0.1 | 19.5 ± 0.5 | 56 |
| TR-MSO-6% | 533 ± 12 | 2.1 ± 0.1 | 9.7 ± 0.2 | 20.0 ± 0.8 | 57 |
| TR-MSO-9% | 504 ± 14 | 2.1 ± 0.2 | 9.2 ± 0.2 | 18.7 ± 1.4 | 58 |
| Property | Sample | |||||
|---|---|---|---|---|---|---|
| 1# 5 phr | 2# 10 phr | 3# 15 phr | 4# 20 phr | 5# 25 phr | 6# TR-AO | |
| Elongation at Break (%) | 371 ± 21 | 407 ± 13 | 411 ± 15 | 503 ± 22 | 533 ± 18 | 542 ± 21 |
| Modulus at 100% (MPa) | 3.6 ± 0.1 | 3.2 ± 0.3 | 2.8 ± 0.1 | 2.4 ± 0.2 | 2.1 ± 0.1 | 2.2 ± 0.1 |
| Modulus at 300% (MPa) | 15.8 ± 0.4 | 14.2 ± 0.1 | 12.7 ± 0.1 | 10.3 ± 0.2 | 9.7 ± 0.1 | 10.0 ± 0.3 |
| Tensile Stress (MPa) | 19.8 ± 0.6 | 19.7 ± 0.4 | 19.5 ± 1.1 | 19.6 ± 0.7 | 19.6 ± 0.7 | 20.4 ± 1.3 |
| Shore A Hardness | 69 | 67 | 63 | 62 | 58 | 59 |
| Akron Abrasion Loss (cm3) | / | / | / | / | 0.1502 | 0.1805 |
| Aging Coefficient (%) | / | / | / | / | 90.74 | 88.74 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Fan, T.; Ye, N.; Wu, W.; Huang, D.; Wang, D.; Wang, Z.; Zhang, L. Plasticization Effect of Bio-Based Plasticizers from Soybean Oil for Tire Tread Rubber. Polymers 2020, 12, 623. https://doi.org/10.3390/polym12030623
Xu H, Fan T, Ye N, Wu W, Huang D, Wang D, Wang Z, Zhang L. Plasticization Effect of Bio-Based Plasticizers from Soybean Oil for Tire Tread Rubber. Polymers. 2020; 12(3):623. https://doi.org/10.3390/polym12030623
Chicago/Turabian StyleXu, Haoshu, Tao Fan, Neng Ye, Weidong Wu, Daye Huang, Danling Wang, Zhao Wang, and Liqun Zhang. 2020. "Plasticization Effect of Bio-Based Plasticizers from Soybean Oil for Tire Tread Rubber" Polymers 12, no. 3: 623. https://doi.org/10.3390/polym12030623