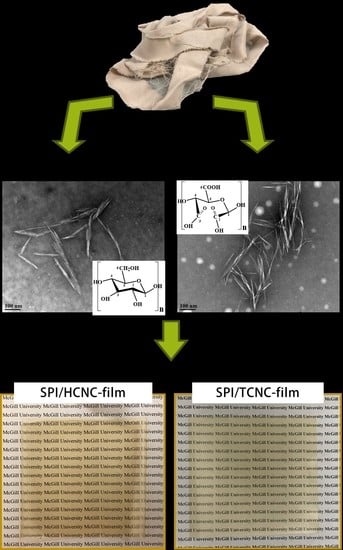
Cellulose Nanocrystals Derived from Textile Waste through Acid Hydrolysis and Oxidation as Reinforcing Agent of Soy Protein Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of CNCs
2.3. Preparation of CNC/SPI Films
2.4. Characterization
2.4.1. Transmission Electron Microscopy (TEM)
2.4.2. X-Ray Diffraction (XRD)
2.4.3. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.4.4. Optical Transmittance Spectra
2.4.5. Water Vapor Permeability (WVP)
2.4.6. Mechanical Properties
2.4.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Racho, P.; Waiwong, W. Modified textile waste for heavy metals removal. Energy Rep. 2020, 6, 927–932. [Google Scholar] [CrossRef]
- Textiles Waste: The Facts. Available online: https://wrwcanada.com/en/get-involved/resources/textiles-themed-resources/textiles-waste-facts (accessed on 4 April 2020).
- Gardetti, M.A.; Muthu, S.S. The UN Sustainable Development Goals for the Textile and Fashion Industry; Springer: Berlin, Germany, 2020. [Google Scholar]
- Cay, A.; Yanik, J.; Akduman, C.; Duman, G.; Ertas, H. Application of textile waste derived biochars onto cotton fabric for improved performance and functional properties. J. Clean Prod. 2020, 251, 119664. [Google Scholar] [CrossRef]
- Xu, Z.; Gu, S.; Sun, Z.; Zhang, D.; Zhou, Y.; Gao, Y.; Qi, R.; Chen, W. Synthesis of char-based adsorbents from cotton textile waste assisted by iron salts at low pyrolysis temperature for Cr(VI) removal. Environ. Sci. Pollut. 2020, 27, 11012–11025. [Google Scholar] [CrossRef] [PubMed]
- Sukhavattanakul, P.; Manuspiya, H. Fabrication of hybrid thin film based on bacterial cellulose nanocrystals and metal nanoparticles with hydrogen sulfide gas sensor ability. Carbohydr. Polym. 2020, 230, 115566. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.T.; Liu, X.H.; Chang, C.Y.; Wang, Y.X. Recent developments and prospective food-related applications of cellulose nanocrystals: A review. Cellulose 2020, 27, 2991–3011. [Google Scholar] [CrossRef]
- Favier, V.; Chanzy, H.; Cavaille, J.Y. Polymer nanocomposites reinforced by cellulose whiskers. Macromolecules 1995, 28, 6365–6367. [Google Scholar] [CrossRef]
- Darpentigny, C.; Molina-Boisseau, S.; Nonglaton, G.; Bras, J.; Jean, B. Ice-templated freeze-dried cryogels from tunicate cellulose nanocrystals with high specific surface area and anisotropic morphological and mechanical properties. Cellulose 2019, 27, 233–247. [Google Scholar] [CrossRef]
- Zhao, G.M.; Du, J.; Chen, W.M.; Pan, M.Z.; Chen, D.Y. Preparation and thermostability of cellulose nanocrystals and nanofibrils from two sources of biomass: Rice straw and poplar wood. Cellulose 2019, 26, 8625–8643. [Google Scholar] [CrossRef]
- Ambrosio-Martin, J.; Fabra, M.J.; Lopez-Rubio, A.; Lagaron, J.M. Melt polycondensation to improve the dispersion of bacterial cellulose into polylactide via melt compounding: Enhanced barrier and mechanical properties. Cellulose 2015, 22, 1201–1226. [Google Scholar] [CrossRef]
- Jiang, Q.; Xing, X.; Jing, Y.; Han, Y. Preparation of cellulose nanocrystals based on waste paper via different systems. Int. J. Biol. Macromol. 2020, 149, 1318–1322. [Google Scholar] [CrossRef]
- Nagarajan, K.J.; Balaji, A.N.; Kasi Rajan, S.T.; Ramanujam, N.R. Preparation of bio-eco based cellulose nanomaterials from used disposal paper cups through citric acid hydrolysis. Carbohydr. Polym. 2020, 235, 115997. [Google Scholar] [CrossRef] [PubMed]
- Sirvio, J.; Hyvakko, U.; Liimatainen, H.; Niinimaki, J.; Hormi, O. Periodate oxidation of cellulose at elevated temperatures using metal salts as cellulose activators. Carbohydr. Polym. 2011, 83, 1293–1297. [Google Scholar] [CrossRef]
- Gao, H.M.; Duan, B.; Lu, A.; Deng, H.B.; Du, Y.M.; Shi, X.W.; Zhang, L.N. Fabrication of cellulose nanofibers from waste brown algae and their potential application as milk thickeners. Food Hydrocolloids 2018, 79, 473–481. [Google Scholar] [CrossRef]
- Gestranius, M.; Stenius, P.; Kontturi, E.; Sjoblom, J.; Tammelin, T. Phase behaviour and droplet size of oil-in-water pickering emulsions stabilised with plant-derived nanocellulosic materials. Colloids Surf. A 2017, 519, 60–70. [Google Scholar] [CrossRef]
- Leung, A.C.W.; Hrapovic, S.; Lam, E.; Liu, Y.L.; Male, K.B.; Mahmoud, K.A.; Luong, J.H.T. Characteristics and properties of carboxylated cellulose nanocrystals prepared from a novel one-step procedure. Small 2011, 7, 302–305. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Xu, W.; Kronlund, D.; Maattanen, A.; Liu, J.; Smatt, J.-H.; Peltonen, J.; Willfor, S.; Mu, X.; Xu, C. Cellulose nanocrystals prepared via formic acid hydrolysis followed by TEMPO-mediated oxidation. Carbohydr. Polym. 2015, 133, 605–612. [Google Scholar] [CrossRef]
- Zhang, Y.; Karimkhani, V.; Makowski, B.; Samaranayake, G.; Rowan, S. Nanoemulsions and nanolatexes stabilized by hydrophobically functionalized cellulose nanocrystals. Macromolecules 2017, 16, 6032–6042. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, Z.; Zhou, J.; Zhang, Y. Reuse of waste cotton cloth for the extraction of cellulose nanocrystals. Carbohydr. Polym. 2017, 157, 945–952. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, X.; Zhang, L. Effects of cellulose whiskers on properties of soy protein thermoplastics. Macromol. Biosci. 2006, 6, 524–531. [Google Scholar] [CrossRef]
- Ko, S.W.; Soriano, J.P.E.; Lee, J.Y.; Unnithan, A.R.; Park, C.H.; Kim, C.S. Nature derived scaffolds for tissue engineering applications: Design and fabrication of a composite scaffold incorporating chitosan-g-d,l-lactic acid and cellulose nanocrystals from Lactuca sativa L. cv green leaf. Int. J. Biol. Macromol. 2018, 110, 504–513. [Google Scholar] [CrossRef]
- Yang, H.; Tejado, A.; Alam, N.; Antal, M.; van de Ven, T.G. Films prepared from electrosterically stabilized nanocrystalline cellulose. Langmuir 2012, 28, 7834–7842. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, L.; Muratore, G.; Ortenzi, M.A.; Gazzotti, S.; Limbo, S.; Piergiovanni, L. Fast production of cellulose nanocrystals by hydrolytic-oxidative microwave-assisted treatment. Polymers 2020, 12, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, C.; Wang, L.; Dong, Y.; Zhang, S.; Shi, S.Q.; Cai, L.; Li, J. Soy protein isolate-based films cross-linked by epoxidized soybean oil. RSC Adv. 2015, 5, 82765–82771. [Google Scholar] [CrossRef]
- Li, K.; Jin, S.; Han, Y.; Li, J.; Chen, H. Improvement in functional properties of soy protein isolate-based film by cellulose nanocrystal-graphene artificial nacre nanocomposite. Polymers 2017, 9, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, H.M.; Wang, C.S.; Zhang, Z.Z.; Yao, S.F. Effect of cellulose nanocrystals on the crystallization behavior and enzymatic degradation of poly(butylene adipate). Carbohydr. Polym. 2018, 189, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Fitch-Vargas, P.R.; Aguilar-Palazuelos, E.; Zazueta-Morales, J.D.; Vega-Garcia, M.O.; Valdez-Morales, J.E.; Martinez-Bustos, F.; Jacobo-Valenzuela, N. Physicochemical and microstructural characterization of corn starch edible films obtained by a combination of extrusion technology and casting technique. J. Food Sci. 2016, 81, E2224–E2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Ma, Y.; Zhao, X.; Ma, D. Development of soybean protein-isolate edible films incorporated with beeswax, span 20, and glycerol. J. Food Sci. 2010, 75, C493–C497. [Google Scholar]
- Yu, H.Y.; Yang, X.Y.; Lu, F.F.; Chen, G.Y.; Yao, J.M. Fabrication of multifunctional cellulose nanocrystals/poly(lactic acid) nanocomposites with silver nanoparticles by spraying method. Carbohydr. Polym. 2016, 140, 209–219. [Google Scholar] [CrossRef]
- Yu, Z.; Sun, L.; Wang, W.; Zeng, W.; Mustapha, A.; Lin, M. Soy protein-based films incorporated with cellulose nanocrystals and pine needle extract for active packaging. Ind. Crops. Prod. 2018, 112, 412–419. [Google Scholar] [CrossRef]
- Abd Hamid, S.B.; Chowdhury, Z.Z.; Karim, M.Z.; Ali, M.E. Catalytic isolation and physicochemical properties of nanocrystalline cellulose (NCC) using HCl-FeCl3 system combined with ultrasonication. BioResources 2016, 11, 3840–3855. [Google Scholar] [CrossRef] [Green Version]
- Beltramino, F.; Roncero, M.B.; Vidal, T.; Valls, C. A novel enzymatic approach to nanocrystalline cellulose preparation. Carbohydr. Polym. 2018, 189, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.C.; Li, C.W.; Chen, F.S. Isolation and characterization of cellulose nanocrystals from cloth hairs and evaluation of their compatibility with PLLA. Cellulose 2017, 24, 4785–4792. [Google Scholar] [CrossRef]
- Hemmati, F.; Jafari, S.M.; Kashaninejad, M.; Barani Motlagh, M. Synthesis and characterization of cellulose nanocrystals derived from walnut shell agricultural residues. Int. J. Biol. Macromol. 2018, 120, 1216–1224. [Google Scholar] [CrossRef]
- Ojala, J.; Sirvio, J.A.; Liimatainen, H. Nanoparticle emulsifiers based on bifunctionalized cellulose nanocrystals as marine diesel oil-water emulsion stabilizers. Chem. Eng. J. 2016, 288, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Marett, J.; Aning, A.; Foster, E.J. The isolation of cellulose nanocrystals from pistachio shells via acid hydrolysis. Ind. Crops Prod. 2017, 109, 869–874. [Google Scholar] [CrossRef]
- Mascheroni, E.; Rampazzo, R.; Ortenzi, M.A.; Piva, G.; Bonetti, S.; Piergiovanni, L. Comparison of cellulose nanocrystals obtained by sulfuric acid hydrolysis and ammonium persulfate, to be used as coating on flexible food-packaging materials. Cellulose 2016, 23, 779–793. [Google Scholar] [CrossRef] [Green Version]
- Lim, W.L.; Gunny, A.A.N.; Kasim, F.H. Overview of cellulose nanocrystals: Extraction, physicochemical properties and applications. IOP Conf. Ser. Mater. Sci. Eng. 2019, 670, 12058. [Google Scholar] [CrossRef]
- Gong, X.; Wang, Y.; Tian, Z.; Zheng, X.; Chen, L. Controlled production of spruce cellulose gels using an environmentally “green” system. Cellulose 2014, 21, 1667–1678. [Google Scholar] [CrossRef]
- Ilharco, L.M.; Garcia, A.R.; daSilva, J.L.; Ferreira, L.F.V. Infrared approach to the study of adsorption on cellulose: Influence of cellulose crystallinity on the adsorption of benzophenone. Langmuir 1997, 13, 4126–4132. [Google Scholar] [CrossRef]
- Lei, W.Q.; Fang, C.Q.; Zhou, X.; Yin, Q.; Pan, S.F.; Yang, R.; Liu, D.H.; Ouyang, Y. Cellulose nanocrystals obtained from office waste paper and their potential application in PET packing materials. Carbohydr. Polym. 2018, 181, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Kafle, K.; Greeson, K.; Lee, C.; Kim, S.H. Cellulose polymorphs and physical properties of cotton fabrics processed with commercial textile mills for mercerization and liquid ammonia treatments. Text Res. J. 2014, 84, 1692–1699. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Medronho, B.F.; Antunes, F.E.; Romano, A.; Miguel, M.G.; Lindman, B. On the role of hydrophobic interactions in cellulose dissolution and regeneration: Colloidal aggregates and molecular solutions. Colloid Surface A 2015, 483, 257–263. [Google Scholar] [CrossRef]
- Gong, X.Y.; Wang, Y.X.; Chen, L.Y. Enhanced emulsifying properties of wood-based cellulose nanocrystals as Pickering emulsion stabilizer. Carbohydr. Polym. 2017, 169, 295–303. [Google Scholar] [CrossRef]
- Ansarifar, E.; Shahidi, F.; Mohebbi, M.; Ramezanian, N.; Koocheki, A.; Mohamadian, A. Optimization of limonene microencapsulation based on native and fibril soy protein isolate by VIKOR method. Lwt-Food Sci. Technol. 2019, 115, 107884. [Google Scholar] [CrossRef]
- Li, K.; Jin, S.; Chen, H.; He, J.; Li, J. A high-performance soy protein isolate-based nanocomposite film modified with microcrystalline cellulose and Cu and Zn nanoclusters. Polymers 2017, 9, 167. [Google Scholar] [CrossRef] [Green Version]
- Carpine, D.; Dagostin, J.L.A.; de Andrade, E.F.; Bertan, L.C.; Mafra, M.R. Effect of the natural surfactant Yucca schidigera extract on the properties of biodegradable emulsified films produced from soy protein isolate and coconut oil. Ind. Crops Prod. 2016, 83, 364–371. [Google Scholar] [CrossRef]
- Cao, J.; Sun, X.; Lu, C.; Zhou, Z.; Zhang, X.; Yuan, G. Water-soluble cellulose acetate from waste cotton fabrics and the aqueous processing of all-cellulose composites. Carbohydr. Polym. 2016, 149, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.; Chiu, F.C. Cellulose nanocrystals reinforced kappa-carrageenan based UV resistant transparent bionanocomposite films for sustainable packaging applications. Carbohydr. Polym. 2019, 211, 181–194. [Google Scholar] [CrossRef]
- Alvarez-Castillo, E.; Bengoechea, C.; Guerrero, A. Composites from by-products of the food industry for the development of superabsorbent biomaterials. Food Bioprod. Process. 2020, 119, 296–305. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L. Cellulose nanowhiskers and fiber alignment greatly improve mechanical properties of electrospun prolamin protein fibers. ACS Appl. Mater. Interfaces 2014, 6, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Song, K.L.; Xing, B.; Hu, W.F.; Ke, Q.F.; Zhao, Y. Thermal-tenacity-enhanced and biodegradable textile sizes from cellulose nanocrystals reinforced soy protein for effective yarn coating. Ind. Crops Prod. 2019, 140, 111701. [Google Scholar] [CrossRef]







| Samples | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) | WVP (10−6 g m−1 h−1 Pa−1) | Transmittance (%, 600 nm) |
|---|---|---|---|---|---|
| SPI film | 2.6 ± 0.42 | 172.15 ± 16.69 | 0.41 ± 0.12 | 1.62 ± 0.15 | 48.7 |
| HCNC-film-10% | 4.66 ± 0.26 | 95.74 ± 3.93 | 1.04 ± 0.10 | 1.35 ± 0.02 | 40.9 |
| HCNC-film-20% | 5.39 ± 0.11 | 69.69 ± 4.80 | 1.12 ± 0.04 | 1.15 ± 0.11 | 37.1 |
| TCNC-film-10% | 7.11 ± 0.35 | 54.06 ± 15.27 | 1.66 ± 0.09 | 1.25 ± 0.02 | 37.0 |
| TCNC-film-20% | 8.81 ± 0.18 | 25.37 ± 5.77 | 4.02 ± 0.34 | 1.15 ± 0.02 | 34.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Tao, R.; Ismail, A.; Wang, Y. Cellulose Nanocrystals Derived from Textile Waste through Acid Hydrolysis and Oxidation as Reinforcing Agent of Soy Protein Film. Polymers 2020, 12, 958. https://doi.org/10.3390/polym12040958
Huang S, Tao R, Ismail A, Wang Y. Cellulose Nanocrystals Derived from Textile Waste through Acid Hydrolysis and Oxidation as Reinforcing Agent of Soy Protein Film. Polymers. 2020; 12(4):958. https://doi.org/10.3390/polym12040958
Chicago/Turabian StyleHuang, Shuting, Ran Tao, Ashraf Ismail, and Yixiang Wang. 2020. "Cellulose Nanocrystals Derived from Textile Waste through Acid Hydrolysis and Oxidation as Reinforcing Agent of Soy Protein Film" Polymers 12, no. 4: 958. https://doi.org/10.3390/polym12040958