Hybrid Polycarbosilane-Siloxane Dendrimers: Synthesis and Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Gel Permeation Chromatography (GPC)
2.2.2. Preparative Chromatography
2.2.3. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.2.4. Gas-liquid Chromatography (GLC)
2.2.5. Differential Scanning Calorimetry (DSC)
2.3. Synthesis of Compounds
2.3.1. Synthesis of the Trimethylsilanol
2.3.2. Synthesis of the 1,1,1,3,5,5,5-heptamethyltrisiloxane
2.3.3. Synthesis of the Carbosilane Dendrimer with the Heptamethyltrisiloxane Shell of the 4th, 6th and 7th Generation (G4(OTMS)32, G6(OTMS)128 and G7(OTMS)256)
2.4. Rheological Studies
2.5. Molecular Dynamics Simulations
2.6. Solution Scattering Experiments and Data Analysis
3. Results and Discussion
3.1. Synthesis of Dendrimers
3.2. Thermophysical Properties
3.3. Rheological Behavior
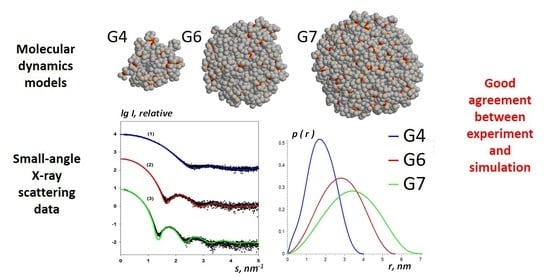
3.4. Structural Modelling
3.5. X-ray Scattering Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frechet, J.M.J.; Tomalia, D.A. Dendrimers and Other Dendritic Polymers, 1st ed.; Wiley: New York, NY, USA, 2002; pp. 1–688. [Google Scholar]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P.A. New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Newkome, G.R.; Moorefield, C.N.; Vogtle, F. Dendritic Macromolecules: Concepts, Syntheses, Perspectives, 1st ed.; Wiley-VCH: Weinheim, Germany, 1996; pp. 1–261. [Google Scholar]
- Ballauff, M.; Likos, C.N. Dendrimers in Solution: Insight from Theory and Simulation. Angew. Chem. Int. Ed. 2004, 43, 2998–3020. [Google Scholar] [CrossRef] [PubMed]
- Vögtle, F.; Richardt, G.; Werner, N. Dendrimer Chemistry: Concepts, Syntheses, properties, Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 1–342. [Google Scholar]
- Voronina, N.V.; Meshkov, I.B.; Myakushev, V.D.; Laptinskaya, T.V.; Papkov, V.S.; Buzin, M.I.; Il’ina, M.N.; Ozerin, A.N.; Muzafarov, A.M. Hybrid Organo-Inorganic Globular Nanospecies: Transition from Macromolecule to Particle. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4310–4322. [Google Scholar] [CrossRef]
- Muzafarov, A.M.; Vasilenko, N.G.; Tatarinova, E.A.; Ignat’eva, G.M.; Myakushev, V.D.; Obrezkova, M.A.; Meshkov, I.B.; Voronina, N.V.; Novozhilov, O.V. Macromolecular nanoobjects as a promising direction of polymer chemistry. Polym. Sci. Ser. C 2011, 53, 48. [Google Scholar] [CrossRef]
- Sheiko, S.S.; Muzafarov, A.M.; Winkler, R.G.; Getmanova, E.V.; Eckert, G.; Reineker, P. Contact Angle Microscopy on a Carbosilane Dendrimer with Hydroxyl End Groups: Method for Mesoscopic Characterization of the Surface Structure. Langmuir 1997, 13, 4172. [Google Scholar] [CrossRef]
- Shumilkina, N.A.; Myakushev, V.D.; Tatarinova, E.A.; Gallyamov, M.O.; Khokhlov, A.R.; Buzin, M.I.; Muzafarov, A.M. Synthesis of a Carbosilane Dendrimer with Fluorocarbon Substituents at the Silicon Atoms in the Surface Layer of the Molecular Structure. Doklady Chem. 2005, 402, 155. [Google Scholar] [CrossRef]
- Kuklin, A.I.; Ignat’eva, G.M.; Ozerina, L.A.; Islamov, A.K.; Mukhamedzyanov, R.I.; Shumilkina, N.A.; Myakushev, V.D.; Sharipov, E.Y.; Gordelii, V.I.; Muzafarov, A.M.; et al. Sans and SAXS study of the structure of organosilicon dendrimers in solutions. Polym. Sci. Ser. A 2002, 44, 1273–1280. [Google Scholar]
- Kuklin, A.I.; Ozerin, A.N.; Islamov, A.K.; Muzafarov, A.M.; Gordeliy, V.I.; Rebrov, E.A.; Ignat’eva, G.M.; Tatarinova, E.A.; Mukhamedzyanov, R.I.; Ozerina, L.A.; et al. Complementarity of small-angle neutron and X-ray scattering methods for the quantitative structural and dynamical specification of dendritic macromolecules. J. Appl. Crystallogr. 2003, 36, 679–683. [Google Scholar] [CrossRef]
- Ozerin, A.N.; Muzafarov, A.M.; Kuklin, A.I.; Islamov, A.K.; Gordelyi, V.I.; Ignat’eva, G.M.; Myakushev, V.D.; Ozerina, L.A.; Tatarinova, E.A. Determination of the Shape of Dendrimer Macromolecules in Solutions from Small-Angle Neutron Scattering Data. Dokl. Chem. 2004, 395, 59–62. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Naylor, A.M.; Goddard III, W.A. Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms to Macroscopic Matter. Angew. Chem. Int. Ed. Engl. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Scherrenberg, R.; Coussens, B.; Van Vliet, P.; Edouard, G.; Brackman, J.; De Brabander, E.; Mortensen, K. Characteristics of poly(propyleneimine) dendrimers as studied with small-angle neutron scattering, viscosimetry, and molecular dynamics. Macromolecules 1998, 31, 456–461. [Google Scholar] [CrossRef]
- Ryabkov, M.V.; Kulagina, T.G.; Lebedev, B.V. The Thermodynamic Properties of Carbosilane Dendrimers of the First and Second Generation with Terminal Allyl Groups in the Temperature Range 0-340 KJ. Russ. J. Phys. Chem. A 2001, 75, 1988–1996. [Google Scholar]
- Lebedev, B.V.; Ryabkov, M.V.; Tatarinova, E.A.; Rebrov, E.A.; Muzafarov, A.M. Thermodynamic Properties of the First to Fifth Generations of Carbosilane Dendrimers with Allyl Terminal Groups. Russ. Chem. Bull. Int. Ed. 2003, 52, 545–551. [Google Scholar] [CrossRef]
- Sagidullin, A.; Muzafarov, A.M.; Krykin, M.A.; Ozerin, A.N.; Skirda, V.D.; Ignat’eva, G.M. Generalized Concentration Dependence of Self-Diffusion Coefficients in Poly(allylcarbosilane) Dendrimer Solutions. Macromolecules 2002, 35, 9472–9479. [Google Scholar] [CrossRef]
- Krykin, M.A.; Volkov, V.I.; Volkov, E.V.; Surin, N.M.; Ozerina, L.A.; Ignat’eva, G.M.; Muzafarov, A.M.; Ozerin, N. Study of Molecular Mobility of Organosilicon Dendritic Macromolecules by Pulsed Field Gradient NMR. Dokl. Chem. 2005, 403, 115–117. [Google Scholar] [CrossRef]
- Lescanec, R.L.; Muthukumar, M. Configurational characteristics and scaling behavior of starburst molecules: A computational study. Macromolecules 1990, 23, 2280–2288. [Google Scholar] [CrossRef]
- Murat, M.; Grest, G.S. Molecular Dynamics Study of Dendrimer Molecules in Solvents of Varying Quality. Macromolecules 1996, 29, 1278–1285. [Google Scholar] [CrossRef]
- Lyulin, A.V.; Davies, G.R.; Adolf, D.B. Brownian Dynamics Simulations of Dendrimers under Shear Flow. Macromolecules 2000, 33, 3294–3304. [Google Scholar] [CrossRef]
- Mazo, M.A.; Zhilin, P.A.; Gusarova, E.B.; Sheiko, S.S.; Balabaev, N.K. Computer Simulation of Intramolecular Mobility of Dendrimers. J. Mol. Liq. 1999, 82, 105–116. [Google Scholar] [CrossRef]
- Klos, J.S.; Sommer, J.U. Coarse grained simulations of neutral and charged dendrimers. Polym. Sci. Ser. C 2013, 55, 125–153. [Google Scholar] [CrossRef]
- Hawker, C.J.; Farrington, P.J.; Mackay, M.E.; Wooley, K.L.; Frechet, J.M.J. Molecular ball bearings: The unusual melt viscosity behavior of dendritic macromolecules. J. Am. Chem. Soc. 1995, 117, 4409–4410. [Google Scholar] [CrossRef]
- Farrington, P.J.; Hawker, C.J.; Frechet, J.M.J.; Mackay, M.E. The melt viscosity of dendritic poly(benzyl ether)Macromolecules. Macromolecules 1998, 31, 5043–5050. [Google Scholar] [CrossRef] [PubMed]
- Sendijarevic, I.; McHugh, A.J. Effects of molecular variables and architecture on the rheological behavior of dendritic polymers. Macromolecules 2000, 33, 590–596. [Google Scholar] [CrossRef]
- Uppuluri, S.; Keinath, S.E.; Tomalia, D.A.; Dvornic, P.R. Rheology of dendrimers. I.Newtonian flow behavior of medium and highly concentrated solutions of polyamidoamine (PAMAM) dendrimers in ethylenediamine (EDA) solvent. Macromolecules 1998, 31, 4498–4510. [Google Scholar] [CrossRef]
- Uppuluri, S.; Morrison, F.A.; Dvornic, P.R. Rheology of Dendrimers. 2. Bulk polyamidoamine dendrimers under steady shear, creep and dynamic oscillatory shear. Macromolecules 2000, 33, 2551–2560. [Google Scholar] [CrossRef]
- Tatarinova, E.A.; Rebrov, E.A.; Myakushev, V.D.; Meshkov, I.B.; Demchenko, N.V.; Bystrova, A.V.; Lebedeva, O.V.; Muzafarov, A.M. Synthesis and study of the properties of the homologous series of polyallylcarbosilane dendrimers and their nonfunctional analogs. Russ. Chem. Bull. 2004, 53, 2591–2600. [Google Scholar] [CrossRef]
- Smirnova, N.N.; Lebedev, B.V.; Khramova, N.M.; Tsvetkova, L.Ya.; Tatarinova, E.A.; Myakushev, V.D.; Muzafarov, A.M. The Thermodynamic Properties of Carbosilane dendrimers of the sixth and seventh generations with terminal allyl groups in the temperature range 6-340 K. Russ. J. Phys. Chem. 2004, 78, 1196–1201. [Google Scholar]
- Smirnova, N.N.; Stepanova, O.V.; Bykova, T.A.; Markin, A.V.; Tatarinova, E.A.; Muzafarov, A.M. Thermodynamic properties of carbosilane dendrimers of the seventh and ninth generations with terminal butyl groups in the temperature range from T→0 to 600 K. Russ. Chem. Bull. 2007, 56, 1991–1995. [Google Scholar] [CrossRef]
- Boldyrev, K.; Tatarinova, E.; Meshkov, I.; Vasilenko, N.; Buzin, M.; Novikov, R.; Vasil’ev, V.; Shtykova, E.; Feigin, L.; Bystrova, A.; et al. New approach to the synthesis of polymethylsilsesquioxane dendrimers. Polymer 2019, 174, 159–169. [Google Scholar] [CrossRef]
- Smirnova, N.N.; Stepanova, O.V.; Bykova, T.A.; Markin, A.V.; Muzafarov, A.M.; Tatarinova, E.A.; Myakushev, V.D. Thermodynamic properties of carbosilane dendrimers of the third to the sixth generations with terminal butyl groups in the range from T→ 0 to 600K. Thermochim. Acta. 2006, 440, 188–194. [Google Scholar] [CrossRef]
- Vasil’ev, V.G.; Kramarenko, E.Yu.; Tatarinova, E.A.; Milenin, S.A.; Kalinina, A.A.; Papkov, V.S.; Muzafarov, A.M. An unprecedented jump in the viscosity of high-generation carbosilane dendrimer melts. Polymer 2018, 146, 1–5. [Google Scholar] [CrossRef]
- Balabaev, N.K.; Mazo, M.A.; Kramarenko, E.Yu. Insight into the structure of polybutylcarbosilane dendrimer melts via extensive molecular dynamics simulations. Macromolecules 2017, 50, 432–445. [Google Scholar] [CrossRef]
- Kurbatov, A.O.; Balabaev, N.K.; Mazo, M.A.; Kramarenko, E.Y. A comparative study of intramolecular mobility of single siloxane and carbosilane dendrimers via molecular dynamics simulations. Polymers 2018, 10, 838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakirov, A.V.; Tatarinova, E.A.; Milenin, S.A.; Shcherbina, M.A.; Muzafarov, A.M.; Chvalun, S.N. Close-packed polybutylcarbosilane dendrimers of higher generations. Soft Matter 2018, 14, 9755–9759. [Google Scholar] [CrossRef] [PubMed]
- Pakula, T.; Vlassopoulos, D.; Fytas, G.; Roovers, J. Structure and Dynamics of Melts of Multiarm Polymer Stars. Macromolecules 1998, 31, 8931–8940. [Google Scholar] [CrossRef]
- Vlassopoulos, D.; Cloitre, M. Tunable rheology of dense soft deformable colloids. Curr. Opin. Colloid Interface Sci. 2014, 19, 561–574. [Google Scholar] [CrossRef]
- Tikhonov, P.A.; Vasilenko, N.G.; Cherkaev, G.V.; Vasil’ev, V.G.; Demchenko, N.V.; Tatarinova, E.A.; Muzafarov, A.M. Synthesis and rheological properties of star-shaped polydimethylsiloxanes based on carbosilane dendrimers. Mendeleev Commun. 2019, 29, 625–627. [Google Scholar] [CrossRef]
- Muzafarov, A.M.; Tatarinova, E.A.; Vasilenko, N.V.; Ignat’eva, G.M. Chapter 8—Organosilicon Dendrimers and Irregular Hyperbranched Polymers. In Organosilicon Compounds Experiment (Physico-Chemical Studies) and Applications, 1st ed.; Lee, V.Y., Ed.; Academic Press: London, UK, 2017; pp. 323–382. [Google Scholar]
- Shishkin, A.N.; Markelov, D.A.; Matveev, V.V. Molecular dynamics simulations of melts of polybutylcarbosilane dendrimers at 600 K. Izv. Akad. Nauk 2016, 1, 67–74. [Google Scholar]
- Ponomarenko, S.A.; Boiko, N.I.; Shibaev, V.P.; Rebrov, E.A.; Muzafarov, A.M. Synthesis of the first-fifth generations of carbosilane liquid-crystalline dendrimers containing terminal cyanobiphenyl groups. Polym. Sci. Ser. A 1998, 40, 763–774. [Google Scholar]
- Ignat’eva, G.M.; Rebrov, E.A.; Myakushev, V.D.; Muzafarov, A.M.; Il’ina, M.N.; Dubovik, I.I.; Papkov, V.S. Polyallylcar-bosilane dendrimers: Synthesis and glass transition. Polymer Sci. Ser. A 1997, 39, 874–881. [Google Scholar]
- Glyakina, A.V.; Balabaev, N.K.; Galzitskaya, O.V. Mechanical unfolding of proteins L and G with constant force: Similarities and differences. J. Chem. Phys. 2009, 131, 045102. [Google Scholar] [CrossRef] [PubMed]
- Lyulin, A.V.; Balabaev, N.K.; Michels, M.A.J. Correlated segmental dynamics in amorphous atactic polystyrene: A molecular dynamics simulation study. Macromolecules 2002, 35, 9595–9604. [Google Scholar] [CrossRef]
- Weiner, S.J.; Kollman, P.A.; Case, D.A.; Chandra Singh, U.; Ghio, C.; Alagona, G.; Profeta, S.; Weiner, P. A new force field for molecular mechanical simulation of nucleic acids and proteins. J. Am. Chem. Soc. 1984, 106, 765–784. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Hill, J.R.; Sauer, J.J. Molecular Mechanics Potential for Silica and Zeolite Catalysts Based on ab Initio Calculations. 2. Aluminosilicates. Phys. Chem. 1995, 99, 9536–9550. [Google Scholar] [CrossRef]
- Lemak, A.S.; Balabaev, N.K. A comparison between collisional dynamics and Brownian dynamics. Mol. Simul. 1995, 15, 223–231. [Google Scholar] [CrossRef]
- Lemak, A.S.; Balabaev, N.K. Molecular dynamics simulation of polymer chain in solution by collisional dynamics method. J. Comput. Chem. 1996, 17, 1685–1695. [Google Scholar] [CrossRef]
- Blanchet, C.E.; Spilotros, A.; Schwemmer, F.; Graewert, M.A.; Kikhney, A.; Jeffries, C.M.; Franke, D.; Mark, D.; Zengerle, R.; Cipriani, F.; et al. Versatile sample environments and automation for biological solution X-ray scattering experiments at the P12 beamline (PETRA III, DESY). J. Appl. Crystallogr. 2015, 48, 431–443. [Google Scholar] [CrossRef] [Green Version]
- Jeffries, C.M.; Graewert, M.A.; Svergun, D.I.; Blanchet, C.E. Limiting radiation damage for high-brilliance biological solution scattering: Practical experience at the EMBL P12 beamline PETRAIII. J. Synchrotron Radiat. 2015, 22, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Feigin, L.A.S.; Svergun, D.I. Structure Analysis by Small-Angle X-Ray and Neutron Scattering, 1st ed.; Plenum Press: New York, NY, USA, 1987; pp. 1–176. [Google Scholar]
- Guinier, A. La diffraction des rayons X aux tres petits angles; application a l’etude de phenomenes ultramicroscopiques. Ann. Phys. 1939, 12, 161–237. [Google Scholar] [CrossRef]
- Porod, G. Chapter 2: General theory. In Small-Angle X-Ray Scattering, 1st ed.; Glatter, O., Kratky, O., Eds.; Academic Press: London, UK, 1982; pp. 17–51. [Google Scholar]
- Svergun, D.I. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 1992, 25, 495–503. [Google Scholar] [CrossRef]
- Svergun, D.I.; Barberato, C.; Koch, M.H.J. CRYSOL—A program to evaluate X-ray solution scattering of biological macromolecules from atomic coordinates. J. Appl. Crystallogr. 1995, 28, 768–773. [Google Scholar] [CrossRef]
- Marciniec, B. Hydrosilylation, A Comprehensive Review on Recent Advances in series Advances in Silicon Science, 1st ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 1–408. [Google Scholar]
- Khairova, R.R.; Milenin, S.A.; Cherkaev, G.V.; Stoikov, I.I.; Muzafarov, A.M. Synthesis of methyl(1-aminophosphonate)siloxane oligomers. Russ. Chem. Bull. 2016, 65, 1285–1288. [Google Scholar] [CrossRef]
- Mironova, M.V.; Semakov, A.V.; Tereshchenko, A.S.; Tatarinova, E.A.; Getmanova, E.V.; Muzafarov, A.M.; Kulichikhin, V.G. Rheology of carbosilane dendrimers with various types of end groups. Polym. Sci. 2010, 52, 1156–1162. [Google Scholar] [CrossRef]
- Tande, B.M.; Wagner, N.J.; Kim, Y.H. Influence of end groups on dendrimer rheology and conformation. Macromolecules 2013, 36, 4619–4623. [Google Scholar] [CrossRef] [Green Version]
- Tatarinova, E.A.; Voronina, N.V.; Bystrova, A.V.; Buzin, M.I.; Muzafarov, A.M. Synthesis and properties of homologous series of polyallylcarbosilane dendrimers with dense macromolecular structure. Macromol. Symp. 2009, 278, 14–23. [Google Scholar] [CrossRef]
- Smirnova, N.N.; Markin, A.V.; Samosudova, Y.S.; Ignat’eva, G.M.; Katarzhnova, E.Y.; Muzafarov, A.M. Thermodynamics of G-3(D4) and G-6(D4) Carbosilanecyclosiloxane Dendrimers. Russ. J. Phys. Chem. A 2013, 87, 552–559. [Google Scholar] [CrossRef]
- Malkin, A. Ya.; Isayev, A.I. Rheology. Concepts, Methods, and Applications, 3rd ed.; ChemTec Publishing: Toronto, ON, Canada, 2017; pp. 1–500. [Google Scholar]







| Type of Terminal Groups | Tg, °C | Ref. |
|---|---|---|
| -SiBu | −94 | [29] |
| -SiAll2 | −100 | [63] |
| -SiMe-[OSiMe2]4-OSiMe3 | −110 | [30] |
| -SiMe[OSiMe3]2 | −83 | |
| -cyclotetrasiloxane | −66 | [64] |
| methylsilsesquioxane dendrimer with terminal -OSiMe3 | −102 | [32] |
| Dendrimer | Intrinsic Viscosity Value [η], dL/g |
|---|---|
| G4(OTMS) | 0.0256 |
| G6(OTMS | 0.0267 |
| G7(OTMS) | 0.0281 |
| Sample | Rg, nm | Dmax, nm | Vp, nm3 |
|---|---|---|---|
| G4 (OTMS) | 1.35 | 4.0 | 17.7 |
| G6 (OTMS) | 2.10 | 5.7 | 64.5 |
| G7 (OTMS) | 2.60 | 7.1 | 117.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milenin, S.A.; Selezneva, E.V.; Tikhonov, P.A.; Vasil’ev, V.G.; Buzin, A.I.; Balabaev, N.K.; Kurbatov, A.O.; Petoukhov, M.V.; Shtykova, E.V.; Feigin, L.A.; et al. Hybrid Polycarbosilane-Siloxane Dendrimers: Synthesis and Properties. Polymers 2021, 13, 606. https://doi.org/10.3390/polym13040606
Milenin SA, Selezneva EV, Tikhonov PA, Vasil’ev VG, Buzin AI, Balabaev NK, Kurbatov AO, Petoukhov MV, Shtykova EV, Feigin LA, et al. Hybrid Polycarbosilane-Siloxane Dendrimers: Synthesis and Properties. Polymers. 2021; 13(4):606. https://doi.org/10.3390/polym13040606
Chicago/Turabian StyleMilenin, Sergey A., Elizaveta V. Selezneva, Pavel A. Tikhonov, Viktor G. Vasil’ev, Alexander I. Buzin, Nikolay K. Balabaev, Andrey O. Kurbatov, Maxim V. Petoukhov, Eleonora V. Shtykova, Lev A. Feigin, and et al. 2021. "Hybrid Polycarbosilane-Siloxane Dendrimers: Synthesis and Properties" Polymers 13, no. 4: 606. https://doi.org/10.3390/polym13040606