Bio-Based Hydrogel and Aerogel Composites Prepared by Combining Cellulose Solutions and Waterborne Polyurethane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cellulose Solutions
2.3. Synthesis of WPU
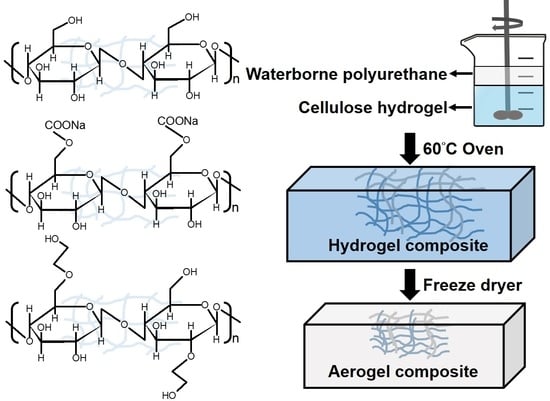
2.4. Preparation and Measurement of Cellulose/WPU Composite Hydrogel
2.5. Manufacturing of Cellulose/WPU Composite Aerogel
2.6. Microstructure of Cellulose/WPU Composite Aerogel
2.7. Water Absorption Capacity of Cellulose/WPU Composite Aerogel
2.8. Thermal Degradation Properties of Cellulose/WPU Composite Aerogel
2.9. Statistical Analyses
3. Results and Discussion
3.1. Properties of WPU Dispersion and Cellulose Solution
3.2. Appearance of Cellulose/WPU Composite Hydrogel
3.3. Physical Properties of Cellulose/WPU Composite Hydrogel
3.4. Compression Properties of Cellulose/WPU Composite Hydrogel
3.5. Microstructure of Cellulose/WPU Composite Aerogel
3.6. Water Absorption Capacity of Cellulose/WPU Composite Hydrogel
3.7. Thermal Resistance of Cellulose/WPU Composite Hydrogel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathur, A.M.; Moorjani, S.K.; Scranton, A.B. Methods for Synthesis of Hydrogel Networks: A Review. J. Macromol. Sci. Part C Polym. Rev. 1996, 36, 405–430. [Google Scholar] [CrossRef]
- Laftah, W.A.; Hashim, S.; Ibrahim, A.N. Polymer Hydrogels: A Review. Polym. Technol. Eng. 2011, 50, 1475–1486. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A Special Material or a New State of Matter: A Review and Reconsideration of the Aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef] [Green Version]
- Stergar, J.; Maver, U. Review of aerogel-based materials in biomedical applications. J. Sol-Gel Sci. Technol. 2016, 77, 738–752. [Google Scholar] [CrossRef]
- Smirnova, I.; Gurikov, P. Aerogel production: Current status, research directions, and future opportunities. J. Supercrit. Fluids 2018, 134, 228–233. [Google Scholar] [CrossRef]
- Ross, P.; Weinhouse, H.; Aloni, Y.; Michaeli, D.; Weinberger-Ohana, P.; Mayer, R.; Braun, S.; De Vroom, E.; Van Der Marel, G.A.; Van Boom, J.H.; et al. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 1987, 325, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, D.; Rudaz, C.; Vasilescu, M.; Budtova, T. Physically and chemically cross-linked cellulose cryogels: Structure, properties and application for controlled release. Carbohydr. Polym. 2016, 151, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Paukkonen, H.; Kunnari, M.; Laurén, P.; Hakkarainen, T.; Auvinen, V.-V.; Oksanen, T.; Koivuniemi, R.; Yliperttula, M.; Laaksonen, T. Nanofibrillar cellulose hydrogels and reconstructed hydrogels as matrices for controlled drug release. Int. J. Pharm. 2017, 532, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.; Kim, D.; Hu, Y.; Kim, Y.; Hong, I.K.; Kim, M.S.; Jung, S. pH-Responsive Succinoglycan-Carboxymethyl Cellulose Hydrogels with Highly Improved Mechanical Strength for Controlled Drug Delivery Systems. Polymers 2021, 13, 3197. [Google Scholar] [CrossRef]
- Jin, C.; Han, S.; Li, J.; Sun, Q. Fabrication of cellulose-based aerogels from waste newspaper without any pretreatment and their use for absorbents. Carbohydr. Polym. 2015, 123, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Ao, C.; Hu, R.; Zhao, J.; Zhang, X.; Li, Q.; Xia, T.; Zhang, W.; Lu, C. Reusable, salt-tolerant and superhydrophilic cellulose hydrogel-coated mesh for efficient gravity-driven oil/water separation. Chem. Eng. J. 2018, 338, 271–277. [Google Scholar] [CrossRef]
- Xie, X.; Liu, L.; Zhang, L.; Lu, A. Strong cellulose hydrogel as underwater superoleophobic coating for efficient oil/water separation. Carbohydr. Polym. 2019, 229, 115467. [Google Scholar] [CrossRef]
- Chen, Y.-C. Thermo and pH-responsive methylcellulose and hydroxypropyl methylcellulose hydrogels containing K2SO4 for water retention and a controlled-release water-soluble fertilizer. Sci. Total Environ. 2018, 655, 958–967. [Google Scholar] [CrossRef]
- Syeda, H.I.; Yap, P.-S. A review on three-dimensional cellulose-based aerogels for the removal of heavy metals from water. Sci. Total Environ. 2021, 807, 150606. [Google Scholar] [CrossRef]
- Teow, Y.H.; Kam, L.M.; Mohammad, A.W. Synthesis of cellulose hydrogel for copper (II) ions adsorption. J. Environ. Chem. Eng. 2018, 6, 4588–4597. [Google Scholar] [CrossRef]
- Shan, S.; Sun, X.-F.; Xie, Y.; Li, W.; Ji, T. High-Performance Hydrogel Adsorbent Based on Cellulose, Hemicellulose, and Lignin for Copper(II) Ion Removal. Polymers 2021, 13, 3063. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.T.; Feng, J.; Ng, S.K.; Wong, J.P.; Tan, V.; Duong, H. Advanced thermal insulation and absorption properties of recycled cellulose aerogels. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 445, 128–134. [Google Scholar] [CrossRef]
- Li, Z.; Shao, L.; Hu, W.; Zheng, T.; Lu, L.; Cao, Y.; Chen, Y. Excellent reusable chitosan/cellulose aerogel as an oil and organic solvent absorbent. Carbohydr. Polym. 2018, 191, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Jeong, D.; Joo, S.-W.; Hu, Y.; Shinde, V.V.; Cho, E.; Jung, S. Carboxymethyl cellulose-based superabsorbent hydrogels containing carboxymehtyl β-cyclodextrin for enhanced mechanical strength and effective drug delivery. Eur. Polym. J. 2018, 105, 17–25. [Google Scholar] [CrossRef]
- Tang, M.; Jia, R.; Kan, H.; Liu, Z.; Yang, S.; Sun, L.; Yang, Y. Kinetic, isotherm, and thermodynamic studies of the adsorption of dye from aqueous solution by propylene glycol adipate-modified cellulose aerogel. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 602, 125009. [Google Scholar] [CrossRef]
- Liang, X.; Qu, B.; Li, J.; Xiao, H.; He, B.; Qian, L. Preparation of cellulose-based conductive hydrogels with ionic liquid. React. Funct. Polym. 2015, 86, 1–6. [Google Scholar] [CrossRef]
- Godiya, C.B.; Cheng, X.; Li, D.; Chen, Z.; Lu, X. Carboxymethyl cellulose/polyacrylamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. J. Hazard. Mater. 2018, 364, 28–38. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Kang, Y.; Wang, A. Synthesis, swelling and responsive properties of a new composite hydrogel based on hydroxyethyl cellulose and medicinal stone. Compos. Part B Eng. 2011, 42, 809–818. [Google Scholar] [CrossRef]
- Verdolotti, L.; Stanzione, M.; Khlebnikov, O.; Silant’Ev, V.; Postnova, I.; Lavorgna, M.; Shchipunov, Y. Dimensionally Stable Cellulose Aerogel Strengthened by Polyurethane Synthesized In Situ. Macromol. Chem. Phys. 2018, 220. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef] [Green Version]
- Lei, W.; Zhou, X.; Fang, C.; Song, Y.; Li, Y. Eco-friendly waterborne polyurethane reinforced with cellulose nanocrystal from office waste paper by two different methods. Carbohydr. Polym. 2019, 209, 299–309. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Fang, C.; Li, S.; Cheng, Y.; Lei, W.; Meng, X. Recent Advances in Synthesis of Waterborne Polyurethane and Their Application in Water-based Ink: A Review. J. Mater. Sci. Technol. 2015, 31, 708–722. [Google Scholar] [CrossRef]
- Lee, W.-J.; Hu, M.-S.; Yu, C.-Y.; Chen, Y.-C. Influences of liquefied lignin content on the properties of waterborne polyurethane prepared with different chain extenders. Holzforschung 2020, 75, 668–676. [Google Scholar] [CrossRef]
- Agnol, L.D.; Dias, F.T.G.; Ornaghi, H.L.; Sangermano, M.; Bianchi, O. UV-curable waterborne polyurethane coatings: A state-of-the-art and recent advances review. Prog. Org. Coat. 2021, 154, 106156. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, Y.; Fan, M.; Jiang, P.; Bao, Y.; Gao, X.; Xia, J. Role of cellulose-based composite materials in synergistic reinforcement of environmentally friendly waterborne polyurethane. Prog. Org. Coat. 2020, 147, 105811. [Google Scholar] [CrossRef]
- Zhai, J.; Zhang, Y.; Cui, C.; Li, A.; Wang, W.; Guo, R.; Qin, W.; Ren, E.; Xiao, H.; Zhou, M. Flexible Waterborne Polyurethane/Cellulose Nanocrystal Composite Aerogels by Integrating Graphene and Carbon Nanotubes for a Highly Sensitive Pressure Sensor. ACS Sustain. Chem. Eng. 2021, 9, 14029–14039. [Google Scholar] [CrossRef]
- Gonzalez, J.S.; Ludueña, L.N.; Ponce, A.; Alvarez, V.A. Poly(vinyl alcohol)/cellulose nanowhiskers nanocomposite hydrogels for potential wound dressings. Mater. Sci. Eng. C 2014, 34, 54–61. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2020, 10, 935–952. [Google Scholar] [CrossRef]
- Karlsson, K.; Schuster, E.; Stading, M.; Rigdahl, M. Foaming behavior of water-soluble cellulose derivatives: Hydroxypropyl methylcellulose and ethyl hydroxyethyl cellulose. Cellulose 2015, 22, 2651–2664. [Google Scholar] [CrossRef]
- Zhang, M.; Ding, C.; Yang, J.; Lin, S.; Chen, L.; Huang, L. Study of interaction between water-soluble collagen and carboxymethyl cellulose in neutral aqueous solution. Carbohydr. Polym. 2016, 137, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Kim, H. Preparation of chitosan aerogel crosslinked in chemical and ionical ways by non-acid condition for wound dressing. Int. J. Biol. Macromol. 2020, 164, 2177–2185. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Abdelgawad, A.M.; Tripathi, A.; Rojas, O.J. Curdlan cryogels reinforced with cellulose nanofibrils for controlled release. J. Environ. Chem. Eng. 2017, 5, 5754–5761. [Google Scholar] [CrossRef]






| Sample Code | Microcrystalline Cellulose | Carboxymethyl Cellulose | Hydroxyethyl Cellulose | Waterborne Polyurethane |
|---|---|---|---|---|
| MC | 100 | - | - | - |
| MC-95/5 | 95 | - | - | 5 |
| MC-90/10 | 90 | - | - | 10 |
| MC-85/15 | 85 | - | - | 15 |
| MC-80/20 | 80 | - | - | 20 |
| CMC | - | 100 | - | - |
| CMC-95/5 | - | 95 | - | 5 |
| CMC-90/10 | - | 90 | - | 10 |
| CMC-85/15 | - | 85 | - | 15 |
| CMC-80/20 | - | 80 | - | 20 |
| HEC | - | - | 100 | - |
| HEC-95/5 | - | - | 95 | 5 |
| HEC-90/10 | - | - | 90 | 10 |
| HEC-85/15 | - | - | 85 | 15 |
| HEC-80/20 | - | - | 80 | 20 |
| Sample | Nonvolatile (%) | pH | Viscosity (cps) | Surface Tension (dyne/cm) |
|---|---|---|---|---|
| WPU | 30.0 | 6.8 | 7 | 40 |
| MC | 8.7 | 12.1 | 56 | 46 |
| CMC | 8.7 | 11.3 | 1378 | 66 |
| HEC | 8.7 | 11.1 | 57 | 42 |
| Sample Code 1 | Apparent Density (g/cm3) | Gel Fraction (%) | Stress (kPa) | |||
|---|---|---|---|---|---|---|
| 5% Strain | 10% Strain | 20% Strain | 40% Strain | |||
| MC | 0.99 ± 0.02 a,2 | 9.50 ± 0.20 a | 0.37 ± 0.01 a,2 | 0.77 ± 0.02 a | 1.90 ± 0.11 a | 9.14 ± 0.89 a |
| MC-95/5 | 0.96 ± 0.03 a | 9.26 ± 0.31 a | 0.26 ± 0.05 a | 0.75 ± 0.10 a | 2.00 ± 0.23 a | 5.63 ± 0.28 bc |
| MC-90/10 | 0.94 ± 0.03 a | 9.01 ± 1.02 a | 0.33 ± 0.07 a | 0.74 ± 0.17 a | 1.94 ± 0.29 a | 7.03 ± 1.61 abc |
| MC-85/15 | 0.95 ± 0.04 a | 9.64 ± 0.30 a | 0.33 ± 0.04 a | 0.76 ± 0.04 a | 2.06 ± 0.11 a | 4.37 ± 0.35 c |
| MC-80/20 | 0.96 ± 0.04 a | 9.41 ± 0.17 a | 0.44 ± 0.11 a | 1.00 ± 0.14 a | 2.58 ± 0.18 a | 7.83 ± 0.18 ab |
| CMC | 1.07 ± 0.10 a’2 | 10.75 ± 0.71 a’ | 0.06 ± 0.00 a’ | 0.10 ± 0.01 a’ | 0.23 ± 0.02 a’ | 0.88 ± 0.14 a’ |
| CMC-95/5 | 1.00 ± 0.02 a’ | 8.68 ± 0.37 c’ | 0.04 ± 0.00 bc’ | 0.07 ± 0.00 ab’ | 0.17 ± 0.02 a’ | 0.76 ± 0.20 a’ |
| CMC-90/10 | 0.96 ± 0.05 a’ | 8.72 ± 0.80 c’ | 0.05 ± 0.01 ab’ | 0.09 ± 0.01 bc’ | 0.22 ± 0.04 a’ | 0.85 ± 0.14 a’ |
| CMC-85/15 | 0.96 ± 0.05 a’ | 9.52 ± 0.20 bc’ | 0.04 ± 0.01 bc’ | 0.08 ± 0.01 abc’ | 0.17 ± 0.03 a’ | 0.82 ± 0.19 a’ |
| CMC-80/20 | 0.94 ± 0.02 a’ | 10.63 ± 0.79 ab’ | 0.04 ± 0.00 c’ | 0.07 ± 0.00 a’ | 0.16 ± 0.00 a’ | 0.62 ± 0.04 a’ |
| HEC | 0.91 ± 0.03 A2 | 7.38 ± 0.37 B | 0.09 ± 0.01 A | 0.17 ± 0.01 A | 0.41 ± 0.03 A | 1.85 ± 0.30 BC |
| HEC-95/5 | 1.02 ± 0.07 A | 8.24 ± 0.43 AB | 0.11 ± 0.00 A | 0.20 ± 0.01 A | 0.45 ± 0.02 A | 1.47 ± 0.05 C |
| HEC-90/10 | 1.01 ± 0.04 A | 7.73 ± 0.35 AB | 0.12 ± 0.01 A | 0.21 ± 0.01 A | 0.50 ± 0.03 A | 2.24 ± 0.15 AB |
| HEC-85/15 | 1.01 ± 0.04 A | 8.80 ± 0.56 A | 0.08 ± 0.00 A | 0.16 ± 0.00 A | 0.38 ± 0.00 A | 1.38 ± 0.10 C |
| HEC-80/20 | 1.01 ± 0.05 A | 8.40 ± 0.15 A | 0.10 ± 0.02 A | 0.19 ± 0.02 A | 0.48 ± 0.02 A | 2.31 ± 0.14 A |
| Sample Code | Onset 1 (°C) | Peak 1 (°C) | End 1 (°C) | Weight Loss (%) |
|---|---|---|---|---|
| WPU | 247 | 367 | 398 | 99 |
| CMC | 241 | 305 | 331 | 61 |
| CMC-95/5 | 263 | 311 | 350 | 65 |
| CMC-90/10 | 272 | 314 | 353 | 63 |
| CMC-85/15 | 260 | 296 | 340 | 66 |
| CMC-80/20 | 252 | 307 | 482 | 70 |
| MC-80/20 | 274 | 340 | 407 | 71 |
| HEC-80/20 | 273 | 347 | 379 | 74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.-J.; Lee, W.-J.; Chen, Y.-C. Bio-Based Hydrogel and Aerogel Composites Prepared by Combining Cellulose Solutions and Waterborne Polyurethane. Polymers 2022, 14, 204. https://doi.org/10.3390/polym14010204
Huang L-J, Lee W-J, Chen Y-C. Bio-Based Hydrogel and Aerogel Composites Prepared by Combining Cellulose Solutions and Waterborne Polyurethane. Polymers. 2022; 14(1):204. https://doi.org/10.3390/polym14010204
Chicago/Turabian StyleHuang, Ling-Jie, Wen-Jau Lee, and Yi-Chun Chen. 2022. "Bio-Based Hydrogel and Aerogel Composites Prepared by Combining Cellulose Solutions and Waterborne Polyurethane" Polymers 14, no. 1: 204. https://doi.org/10.3390/polym14010204