Exploration of a Chemo-Mechanical Technique for the Isolation of Nanofibrillated Cellulosic Fiber from Oil Palm Empty Fruit Bunch as a Reinforcing Agent in Composites Materials
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
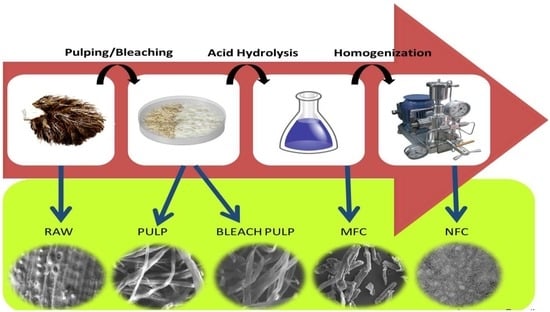
2.2. Preparation of Cellulose Fiber
2.3. Preparation of Nanofibrillated Cellulose Fiber
2.4. Characterization
2.4.1. Morphology Analysis
2.4.2. Fourier Transform Infrared Spectroscopy
2.4.3. X-ray Diffraction Analysis
2.4.4. Thermogravimetric Analysis
3. Results and Discussion
3.1. Morphological Observation



3.2. FT-IR Spectroscopy

3.3. X-ray Diffraction


3.4. Thermogravimetric Analysis (TGA)

| EFB fibre | IDT (°C) | FDT (°C) | Tmax (°C) | Residue (%) |
|---|---|---|---|---|
| Raw | 264 ± 2.14 | 389 ± 2.42 | 330 ± 2.34 | 19.90 ± 0.50 |
| Pulp | 308 ± 2.32 | 389 ± 2.36 | 349 ± 2.26 | 7.28 ± 0.21 |
| Bleached pulp | 308 ± 2.41 | 379 ± 2.18 | 354 ± 2.49 | 9.10 ± 0.16 |
| MFC | 253 ± 2.73 | 389 ± 2.54 | 326 ± 2.12 | 14.08 ± 0.30 |
| NFC | 300 ± 2.43 | 379 ± 2.61 | 357 ± 2.48 | 5.01 ± 0.04 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abdul Khalil, H.P.S.; Bhat, A.H.; Ireana Yusra, A.F. Green composites from sustainable cellulose nanofibrils: A review. Carbohyd. Polym. 2012, 87, 963–979. [Google Scholar]
- Jawaid, M.; Othman, A.N.; Saba, Y.A.S.; Paridah, M.T.; Khalil, H.P.S.A. Effect of chemical modifications of fibres on tensile properties of epoxy hybrid composites. Int. J. Polym. Anal. Charact. 2014, 19, 62–69. [Google Scholar] [CrossRef]
- Fahma, F.; Iwamoto, S.; Hori, N.; Iwata, T.; Takemura, A. Isolation, preparation, and characterization of nanofibers from oil palm empty-fruit-bunch (opefb). Cellulose 2010, 17, 977–985. [Google Scholar] [CrossRef]
- Fahma, F.; Iwamoto, S.; Hori, N.; Iwata, T.; Takemura, A. Effect of pre-acid-hydrolysis treatment on morphology and properties of cellulose nanowhiskers from coconut husk. Cellulose 2011, 18, 443–450. [Google Scholar] [CrossRef]
- Nazir, M.S.; Wahjoedi, B.A.; Yussof, A.W.; Abdullah, M.A. Eco-friendly extraction and characterization of cellulose from oil palm empty fruit bunches. Bioresources 2013, 8, 2161–2172. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohyd. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef]
- Siddiqui, N.; Mills, R.H.; Gardner, D.J.; Bousfield, D. Production and characterization of cellulose nanofibers from wood pulp. J. Adhes. Sci. Technol. 2011, 25, 709–721. [Google Scholar] [CrossRef]
- Jonoobi, M.; Khazaeian, A.; Tahir, P.M.; Azry, S.S.; Oksman, K. Characteristics of cellulose nanofibers isolated from rubberwood and empty fruit bunches of oil palm using chemo-mechanical process. Cellulose 2011, 18, 1085–1095. [Google Scholar] [CrossRef]
- Chan, H.C.; Chia, C.H.; Zakaria, S.; Ahmad, I.; Dufresne, A. Production and characterisation of cellulose and nano-crystalline cellulose from kenaf core wood. BioResources 2012, 8, 785–794. [Google Scholar] [CrossRef]
- Yu, M.; Yang, R.; Huang, L.; Cao, X.; Yang, F.; Liu, D. Preparation and characterization of bamboo nanocrystalline cellulose. BioResources 2012, 7, 1802–1812. [Google Scholar]
- Qua, E.H.; Hornsby, P.R.; Sharma, H.S.S.; Lyons, G. Preparation and characterisation of cellulose nanofibres. J. Mater. Sci. 2011, 46, 6029–6045. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Mahayuni, R.; Bhat, I.U.H.; Rudi, D.; Almulali, M.Z.; Abdullah, C.K. Characterization of various organic waste nanofillers obtained from oil palm ash. BioResources 2012, 7, 5771–5780. [Google Scholar]
- Lu, P.; Hsieh, Y.L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohyd. Polym. 2012, 87, 564–573. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Tahir, P.M.; Shakeri, A.; SaifulAzry, S.; Makinejad, M.D. Physicochemical characterization of pulp and nanofibers from kenaf stem. Mater. Lett. 2011, 65, 1098–1100. [Google Scholar] [CrossRef]
- Turunawarasua, D.; Chana, Y.P.; Muhaiminb, W.P.A.W.; Honb, L.H.; Syedb, O.B.; Nguyenb, T.H.V.; Borhanb, A.B. Thermo-economic analysis of a novel conceptual process model for sustainable power plants using empty fruit bunches. Int. J. Chem. Environ. Eng. 2013, 4, 310–315. [Google Scholar]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohyd. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstrom, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Lasseuguette, E.; Roux, D.; Nishiyama, Y. Rheological properties of microfibrillar suspension of tempo-oxidized pulp. Cellulose 2008, 15, 425–433. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D.; Hedenqvist, M.; Ankerfors, M.; Lindström, T. Highly transparent films from carboxymethylated microfibrillated cellulose: The effect of multiple homogenization steps on key properties. J. Appl. Polym. Sci. 2011, 119, 2652–2660. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Shakeri, A.; Misra, M.; Oksman, K. Chemical composition, crystallinity, and thermal degradation of bleached and unbleached kenaf bast (Hibiscus cannabinus) pulp and nanofibers. BioResources 2009, 4, 626–639. [Google Scholar]
- Iwamoto, S.; Nakagaito, A.N.; Yano, H. Nano-fibrillation of pulp fibers for the processing of transparent nanocomposites. Appl. Phys. A Mater. 2007, 89, 461–466. [Google Scholar] [CrossRef]
- Pan, M.; Zhou, X.; Chen, M. Cellulose nanowhiskers isolation and properties from acid hydrolysis combined with high pressure homogenization. BioResources 2013, 8, 933–943. [Google Scholar] [CrossRef]
- Wan Rosli, W.D.; Leh, C.P.; Zainuddin, Z.; Tanaka, R. Optimization of soda pulping variable for preparation of dissolving pulps from oil palm fiber. Holzforschung 2003, 57, 106–114. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Yusra, A.F.I.; Bhat, A.H.; Jawaid, M. Cell wall ultrastructure, anatomy, lignin distribution, and chemical composition of Malaysian cultivated kenaf fiber. Ind. Crop. Prod. 2010, 31, 113–121. [Google Scholar]
- Haafiz, M.K.M.; Eichhorn, S.J.; Hassan, A.; Jawaid, M. Isolation and characterization of microcrystalline cellulose from oil palm biomass residue. Carbohyd. Polym. 2013, 93, 628–634. [Google Scholar] [CrossRef] [Green Version]
- Ciolacu, D.; Ciolacu, F.; Popa, V. Amorphous cellulose-structure and characterization. Cell. Chem. Technol. 2011, 45, 13–21. [Google Scholar]
- Jahan, M.S.; Saeed, A.; He, Z.B.; Ni, Y.H. Jute as raw material for the preparation of microcrystalline cellulose. Cellulose 2011, 18, 451–459. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatah, I.Y.A.; Khalil, H.P.S.A.; Hossain, M.S.; Aziz, A.A.; Davoudpour, Y.; Dungani, R.; Bhat, A. Exploration of a Chemo-Mechanical Technique for the Isolation of Nanofibrillated Cellulosic Fiber from Oil Palm Empty Fruit Bunch as a Reinforcing Agent in Composites Materials. Polymers 2014, 6, 2611-2624. https://doi.org/10.3390/polym6102611
Fatah IYA, Khalil HPSA, Hossain MS, Aziz AA, Davoudpour Y, Dungani R, Bhat A. Exploration of a Chemo-Mechanical Technique for the Isolation of Nanofibrillated Cellulosic Fiber from Oil Palm Empty Fruit Bunch as a Reinforcing Agent in Composites Materials. Polymers. 2014; 6(10):2611-2624. https://doi.org/10.3390/polym6102611
Chicago/Turabian StyleFatah, Ireana Yusra A., H. P. S. Abdul Khalil, Md. Sohrab Hossain, Astimar A. Aziz, Yalda Davoudpour, Rudi Dungani, and Amir Bhat. 2014. "Exploration of a Chemo-Mechanical Technique for the Isolation of Nanofibrillated Cellulosic Fiber from Oil Palm Empty Fruit Bunch as a Reinforcing Agent in Composites Materials" Polymers 6, no. 10: 2611-2624. https://doi.org/10.3390/polym6102611