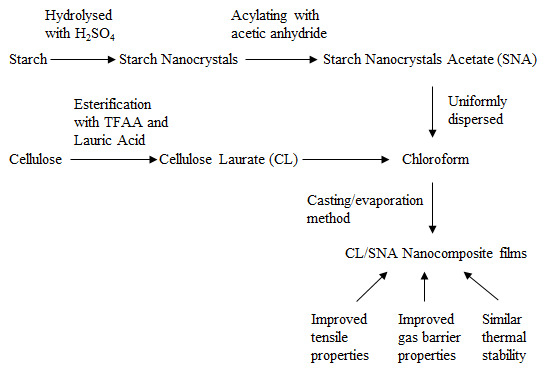
Preparation and Properties of Cellulose Laurate (CL)/Starch Nanocrystals Acetate (SNA) Bio-nanocomposites
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Fabrication of SNs
2.3. Synthesis of SNA
2.4. Preparation of CL/SNA Nanocomposite Films
2.5. Analytical Methods
2.5.1. Fourier Transform Infrared Spectrometer (FTIR)
2.5.2. Characterization of SNs Particles
2.5.3. Light Transmittance of CL/SNA Nanocomposite Films
2.5.4. Thermogravimetric Analysis
2.5.5. Tensile Analysis
2.5.6. Dynamic Mechanical Analysis (DMA)
2.5.7. Contact Angles Measurements
2.5.8. Barrier Properties Measurements
3. Results and Discussion
3.1. Synthesis and Characterization of SNA
3.1.1. FTIR

3.1.2. Dispersion in Different Solvents and Particles Distribution of SNs and SNA


3.2. Properties of CL/SNA Nanocomposite Films
3.2.1. Light Transmittance of CL/SNA Nanocomposite Films

3.2.2. Thermogravimetric Analysis (TG)

3.2.3. Mechanical Properties
Tensile Tests

| Sample | σb/MPa | E/MPa | εb/% |
|---|---|---|---|
| CL | 59.53 ± 4.56 | 200.08 ± 10.27 | 36.5 ± 2.14 |
| CL/SNA01 | 58.86 ± 5.71 | 267.77 ± 14.31 | 37.7 ± 3.52 |
| CL/SNA03 | 60.02 ± 6.23 | 310.30 ± 10.89 | 28.3 ± 1.89 |
| CL/SNA05 | 65.41 ± 7.85 | 330.09 ± 22.14 | 28.5 ± 3.26 |
| CL/SNA07 | 65.83 ± 5.34 | 335.49 ± 19.78 | 11.8 ± 1.08 |
| CL/SNA10 | 67.17 ± 2.11 | 338.57 ± 25.31 | 8.1 ± 1.13 |
Dynamic Mechanical Analysis (DMA)

3.2.4. Contact Angles of CL/SNA Nanocomposite Films

3.2.5. Barrier Properties of CL/SNA Nanocomposite Films
| Sample | WVP (×10−6 g·cm·cm−2·s−1·Pa−1) | PO2 (×10−14 mL·cm·cm−2·s−1·Pa−1) |
|---|---|---|
| CL | 8.85 ± 0.08 | 7.08 ± 0.07 |
| CL/SNA01 | 8.71 ± 0.05 | 6.98 ± 0.11 |
| CL/SNA03 | - | - |
| CL/SNA05 | 7.34 ± 0.10 | 6.52 ± 0.08 |
| CL/SNA07 | 7.36 ± 0.11 | 6.41 ± 0.13 |
| CL/SNA10 | 7.08 ± 0.06 | 6.35 ± 0.05 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Filho, G.R.; Monteiro, D.S.; Meireles, C.S.; Nascimento de Assuncao, R.M.; Cerqueira, D.A.; Barud, H.S.; Ribeiro, S.J.L.; Messadeq, Y. Synthesis and characterization of cellulose acetate produced from recycled newspaper. Carbohydr. Polym. 2008, 73, 74–82. [Google Scholar] [CrossRef]
- Huang, F.Y. Thermal properties and thermal degradation of cellulose tri-stearate (CTs). Polymers 2012, 4, 1012–1024. [Google Scholar] [CrossRef]
- Kwatra, H.S.; Caruthers, J.M.; Tao, B.Y. Synthesis of long chain fatty acids esterified onto cellulose via the vacuum-acid chloride process. Ind. Eng. Chem. Res. 1992, 31, 2647–2651. [Google Scholar] [CrossRef]
- Crepy, L.; Miri, V.; Joly, N.; Martin, P.; Lefebvre, J.M. Effect of side chain length on structure and thermomechanical properties of fully substituted cellulose fatty esters. Carbohydr. Polym. 2011, 83, 1812–1820. [Google Scholar] [CrossRef]
- Granstrom, M.; nee Paakko, M.K.; Jin, H.; Kolehmainen, E.; Kilpelainen, I.; Ikkala, O. Highly water repellent aerogels based on cellulose stearoyl esters. Polym. Chem. 2011, 2, 1789–1796. [Google Scholar] [CrossRef]
- Crepy, L.; Monchau, F.; Chai, F.; Raoul, G.; Hivart, P.; Hildebrand, H.F.; Martin, P.; Joly, N. Evaluation of a bio-based hydrophobic cellulose laurate film as biomaterial—Study on biodegradation and cytocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Crépy, L.; Chaveriat, L.; Banoub, J.; Martin, P.; Joly, N. Synthesis of cellulose fatty esters as plastics—Influence of the degree of substitution and the fatty chain length on mechanical properties. ChemSusChem 2009, 2, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Sealey, J.E.; Samaranayake, G.; Todd, J.G.; Glasser, W.G. Novel cellulose derivatives. IV. Preparation and thermal analysis of waxy esters of cellulose. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 1613–1620. [Google Scholar] [CrossRef]
- Geissler, A.; Bonaccurso, E.; Heim, L.O.; Heinze, T.; Zhang, K. Temperature-responsive thin films from cellulose stearoyl triester. J. Phys. Chem. C 2014, 118, 2408–2417. [Google Scholar] [CrossRef]
- Dufresne, A. Crystalline starch based nanoparticles. Curr. Opin. Colloid Interface Sci. 2014, 19, 397–408. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nano-composites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sahoo, B.; Teraoka, I.; Miller, L.M.; Gross, R.A. Enzyme-catalyzed regioselective modification of starch nanoparticles. Macromolecules 2005, 38, 61–68. [Google Scholar] [CrossRef]
- Thielemans, W.; Belgacem, M.N.; Dufresne, A. Starch nanocrystals with large chain surface modifications. Langmuir 2006, 22, 4804–4810. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A.; Cavaille, J.Y. Clustering and percolation effects in microcrystalline starch-reinforced thermoplastic. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 2211–2224. [Google Scholar] [CrossRef]
- Angellier, H.; Molina-Boisseau, S.; Dufresne, A. Processing and structural properties of waxy maize starch nanocrystals reinforced natural rubber. Macromolecules 2005, 38, 9161–9170. [Google Scholar] [CrossRef]
- Chen, G.; Wei, M.; Chen, J.; Huang, J.; Dufresne, A.; Chang, P.R. Simultaneous reinforcing and toughening: New nanocomposites of waterborne polyurethane filled with low loading level of starch nanocrystals. Polymer 2008, 49, 1860–1870. [Google Scholar] [CrossRef]
- Angellier, H.; Molina-Boisseau, S.; Dole, P.; Dufresne, A. Thermoplastic starch—Waxy maize starch nanocrystals nanocomposites. Biomacromolecules 2006, 7, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.L.; Ribba, L.; Dufresne, A.; Aranguren, M.I.; Goyanes, S. Physico-mechanical properties of biodegradable starch nanocomposites. Macromol. Mater. Eng. 2009, 294, 169–177. [Google Scholar] [CrossRef]
- Viguie, J.; Molina-Boisseau, S.; Dufresne, A. Processing and characterization of waxy maize starch films plasticized by sorbitol and reinforced with starch nanocrystals. Macromol. Biosci. 2007, 7, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Kristo, E.; Biliaderis, C.G. Physical properties of starch nanocrystal-reinforced pullulan films. Carbohydr. Polym. 2007, 68, 146–158. [Google Scholar] [CrossRef]
- Yu, J.; Ai, F.; Dufresne, A.; Gao, S.; Huang, J.; Chang, P.R. Structure and mechanical properties of poly(lactic acid) filled with (starch nanocrystal)-graft-poly(ε-caprolactone). Macromol. Mater. Eng. 2008, 293, 763–770. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, X.; Chang, P.R.; Huneault, M.A. Comparative study on the films of poly(vinyl alcohol)/pea starch nanocrystals and poly(vinyl alcohol)/native pea starch. Carbohydr. Polym. 2008, 73, 8–17. [Google Scholar] [CrossRef]
- Angellier, H.; Choisnard, L.; Molina-Boisseau, S.; Ozil, P.; Dufresne, A. Optimization of the preparation of aqueous suspensions of waxy maize starch nanocrystals using a response surface methodology. Biomacromolecules 2004, 5, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- LeCorre, D.; Dufresne, A.; Rueff, M.; Khelifi, B.; Bras, J. All starch nanocomposite coating for barrier material. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Namazi, H.; Dadkhah, A. Surface modification of starch nanocrystals through ring-opening polymerization of ε-caprolactone and investigation of their microstructures. J. Appl. Polym. Sci. 2008, 110, 2405–2412. [Google Scholar] [CrossRef]
- Yano, H.; Sugiyama, J.; Nakagaito, A.N.; Nogi, M.; Matsuura, T.; Hikita, M.; Handa, K. Optically transparent composites reinforced with networks of bacterial nanofibers. Adv. Mater. 2005, 17, 153–155. [Google Scholar] [CrossRef]
- Nogi, M.; Iwamoto, S.; Nakagaito, A.N.; Yano, H. Optically transparent nanofiber paper. Adv. Mater. 2009, 21, 1595–1598. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Wang, W.J.; Shao, Z.Q.; Zhu, H.D.; Li, Y.H.; Wang, F.J. The transparency and mechanical properties of cellulose acetate nanocomposites using cellulose nanowhiskers as fillers. Cellulose 2013, 20, 159–168. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiu, C.; Ji, N.; Sun, C.; Xiong, L.; Sun, Q. Mechanical, barrier and morphological properties of starch nanocrystals-reinforced pea starch films. Carbohydr. Polym. 2015, 121, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Mele, P.; Angellier-Coussy, H.; Molina-Boisseau, S.; Dufresne, A. Reinforcing mechanisms of starch nanocrystals in a nonvulcanized natural rubber matrix. Biomacromolecules 2011, 12, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Vaca-Garcia, C.; Gozzelino, G.; Glasser, W.G.; Borredon, M.E. Dynamic mechanical thermal analysis transitions of partially and fully substituted cellulose fatty esters. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 281–288. [Google Scholar] [CrossRef]
- Liu, D.; Zhong, T.; Chang, P.R.; Li, K.; Wu, Q. Starch composites reinforced by bamboo cellulosic crystals. Bioresour. Technol. 2010, 101, 2529–2536. [Google Scholar] [CrossRef] [PubMed]
- Kargarzadeh, H.; Sheltami, R.M.; Ahmada, I.; Abdullaha, I.; Dufresne, A. Toughened polyester cellulose nanocomposites: Effects of cellulose nanocrystals and liquid epoxidized natural rubber on morphology and mechanical properties. Ind. Crops Prod. 2015, 72, 125–132. [Google Scholar] [CrossRef]
- Aldana, D.S.; Villa, E.D.; de Dios Hernández, M.; Sánchez, G.G.; Cruz, Q.R.; Gallardo, S.F.; Castillo, H.P.; Casarrubias, L.B. Barrier properties of polylactic acid in cellulose based packages using montmorillonite as filler. Polymers 2014, 6, 2386–2403. [Google Scholar] [CrossRef]
- Condes, M.C.; Anon, M.C.; Mauri, A.N.; Dufresne, A. Amaranth protein films reinforced with maize starch nanocrystals. Food Hydrocoll. 2015, 47, 146–157. [Google Scholar] [CrossRef]
- Dou, Y.; Xu, S.; Liu, X.; Han, J.; Yan, H.; Wei, M.; Evans, D.G.; Duan, X. Transparent, flexible films based on layered double hydroxide/cellulose acetate with excellent oxygen barrier property. Adv. Funct. Mater. 2014, 24, 514–521. [Google Scholar] [CrossRef]
- Yang, Q.; Saito, T.; Isogai, A. Facile fabrication of transparent cellulose films with high water repellency and gas barrier properties. Cellulose 2012, 19, 1913–1921. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, F.-Y.; Wu, X.-J.; Yu, Y.; Lu, Y.-H. Preparation and Properties of Cellulose Laurate (CL)/Starch Nanocrystals Acetate (SNA) Bio-nanocomposites. Polymers 2015, 7, 1331-1345. https://doi.org/10.3390/polym7071331
Huang F-Y, Wu X-J, Yu Y, Lu Y-H. Preparation and Properties of Cellulose Laurate (CL)/Starch Nanocrystals Acetate (SNA) Bio-nanocomposites. Polymers. 2015; 7(7):1331-1345. https://doi.org/10.3390/polym7071331
Chicago/Turabian StyleHuang, Feng-Yuan, Xiao-Jie Wu, Yan Yu, and Yan-Hua Lu. 2015. "Preparation and Properties of Cellulose Laurate (CL)/Starch Nanocrystals Acetate (SNA) Bio-nanocomposites" Polymers 7, no. 7: 1331-1345. https://doi.org/10.3390/polym7071331