Flammability of Cellulose-Based Fibers and the Effect of Structure of Phosphorus Compounds on Their Flame Retardancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Flame Retardants
2.1.1. Synthesis of Mono-Substituted Phosphoramidates (General Procedure)
2.1.2. Synthesis of Bis-Phosphoramidates
Synthesis of EDA-DEP
Synthesis of EDA-DMP
2.2. Treatments of Flame Retardants and Elemental Analysis
2.3. Fire Test
2.4. Thermal Analysis
3. Results and Discussion
3.1. Choice of Cellulose Materials
3.2. Chemical Composition of Cellulose Textiles
3.3. Burning Behavior of Cellulose Textiles and its Thermal Analysis
3.4. Choice of Phosphoramidates and Application on Cellulose Textiles
3.5. Thermal and Calorimetry Data of Treated Cellulose Fibers
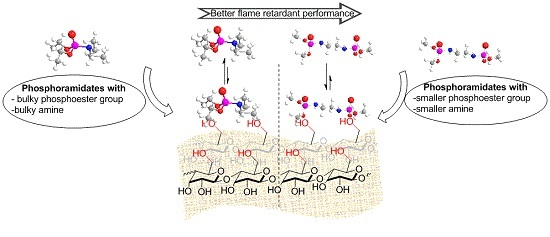
3.6. FR Structure-FR Efficacy: Possible Mechanism
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kozłowski, R.; Władyka-Przybylak, M. Flammability and fire resistance of composites reinforced by natural fibers. Polym. Adv. Technol. 2008, 19, 446–453. [Google Scholar] [CrossRef]
- Kabir, M.M.; Wang, H.; Lau, K.T.; Cardona, F. Chemical treatments on plant-based natural fibre reinforced polymer composites: An overview. Compos. Part B 2012, 43, 2883–2892. [Google Scholar] [CrossRef]
- Dorez, G.; Ferry, L.; Sonnier, R.; Taguet, A.; Lopez-Cuesta, J.M. Effect of cellulose, hemicellulose and lignin contents on pyrolysis and combustion of natural fibers. J. Anal. Appl. Pyrolysis 2014, 107, 323–331. [Google Scholar] [CrossRef]
- Hosoya, T.; Kawamoto, H.; Saka, S. Role of methoxyl group in char formation from lignin-related compounds. J. Anal. Appl. Pyrolysis 2009, 84, 79–83. [Google Scholar] [CrossRef]
- Dittenber, D.B.; GangaRao, H.V.S. Critical review of recent publications on use of natural composites in infrastructure. Compos. Part A 2012, 43, 1419–1429. [Google Scholar] [CrossRef]
- Matkó, S.; Toldy, A.; Keszei, S.; Anna, P.; Bertalan, G.; Marosi, G. Flame retardancy of biodegradable polymers and biocomposites. Polym. Degrad. Stab. 2005, 88, 138–145. [Google Scholar] [CrossRef]
- Gaan, S.; Sun, G. Effect of phosphorus and nitrogen on flame retardant cellulose: A study of phosphorus compounds. J. Anal. Appl. Pyrolysis 2007, 78, 371–377. [Google Scholar] [CrossRef]
- Gaan, S.; Sun, G. Effect of nitrogen additives on thermal decomposition of cotton. J. Anal. Appl. Pyrolysis 2009, 84, 108–115. [Google Scholar] [CrossRef]
- Gaan, S.; Sun, G.; Hutches, K.; Engelhard, M.H. Effect of nitrogen additives on flame retardant action of tributyl phosphate: Phosphorus-nitrogen synergism. Polym. Degrad. Stab. 2008, 93, 99–108. [Google Scholar] [CrossRef]
- Gaan, S.; Viktoriya, S.; Ottinger, S.; Heuberger, M.; Ritter, A. Phosphoramidate Flame Retardants. WO2009153034A1, 23 December 2009. [Google Scholar]
- Rupper, P.; Gaan, S.; Salimova, V.; Heuberger, M. Characterization of chars obtained from cellulose treated with phosphoramidate flame retardants. J. Anal. Appl. Pyrolysis 2010, 87, 93–98. [Google Scholar] [CrossRef]
- Salimova, V.; Dimitry, N.; Gaan, S. Effect of chemical environment of organophosphorus compounds on thermal decomposition of cellulose. PMSE Prepr. 2008, 98, 250–251. [Google Scholar]
- Gaan, S.; Rupper, P.; Salimova, V.; Heuberger, M.; Rabe, S.; Vogel, F. Thermal decomposition and burning behavior of cellulose treated with ethyl ester phosphoramidates: Effect of alkyl substituent on nitrogen atom. Polym. Degrad. Stab. 2009, 94, 1125–1134. [Google Scholar] [CrossRef]
- Horrocks, A.R. Flame retardant challenges for textiles and fibres: New chemistry versus innovatory solutions. Polym. Degrad. Stab. 2011, 96, 377–392. [Google Scholar] [CrossRef]
- Schmidt, E.G. Process for Preparing Peat Fibers from Peat. WO8301445A1, 28 April 1983. [Google Scholar]
- Sepulveda, L.; Troncoso, F.; Contreras, E.; Palma, C. Competitive adsorption of textile dyes using peat: Adsorption equilibrium and kinetic studies in monosolute and bisolute systems. Environ. Technol. 2008, 29, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Suni, S.; Kosunen, A.L.; Romantschuk, M. Microbially treated peat-cellulose fabric as a biodegradable oil-collection cloth. J. Environ. Sci. Health Part A 2006, 41, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Zaharia, C. Application of waste materials as “low cost” sorbents for industrial effluent treatment: A comparative overview. Int. J. Mater. Prod. Technol. 2015, 50, 196–220. [Google Scholar] [CrossRef]
- Pinto, P.C.; Evtuguin, D.V.; Neto, C.P. Structure of hardwood glucuronoxylans: Modifications and impact on pulp retention during wood kraft pulping. Carbohydr. Polym. 2005, 60, 489–497. [Google Scholar] [CrossRef]
- Gaan, S.; Sun, G. Effect of phosphorus flame retardants on thermo-oxidative decomposition of cotton. Polym. Degrad. Stab. 2007, 92, 968–974. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Fage, J.; Liang, S.; Gaan, S. An overview of mode of action and analytical methods for evaluation of gas phase activities of flame retardants. Polymers 2015, 7, 504–526. [Google Scholar] [CrossRef]
- Jeler, S.; Kresevic, B.; Golob, V. Effect of apparent fabric density and pore volume on LOI (limiting oxygen index). Textilveredlung 1985, 20, 158–160. [Google Scholar]
- Ozcan, G.; Dayioglu, H.; Candan, C. Effect of gray fabric properties on flame resistance of knitted fabric. Text. Res. J. 2003, 73, 883–891. [Google Scholar] [CrossRef]
- Gallo, J.M.; Almirall, J.R. Elemental analysis of white cotton fiber evidence using solution ICP-MS and laser ablation ICP-MS (LA-ICP-MS). Forensic. Sci. Int. 2009, 190, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Degani, O.; Gepstein, S.; Dosoretz, C.G. A new method for measuring scouring efficiency of natural fibers based on the cellulose-binding domain-β-glucuronidase fused protein. J. Biotechnol. 2004, 107, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Qu, T.; Guo, W.; Shen, L.; Xiao, J.; Zhao, K. Experimental study of biomass pyrolysis based on three major components: Hemicellulose, cellulose, and lignin. Ind. Eng. Chem. Res. 2011, 50, 10424–10433. [Google Scholar] [CrossRef]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef]
- Gani, A.; Naruse, I. Effect of cellulose and lignin content on pyrolysis and combustion characteristics for several types of biomass. Renew. Energy 2007, 32, 649–661. [Google Scholar] [CrossRef]
- Yang, C.Q.; He, Q.; Lyon, R.E.; Hu, Y. Investigation of the flammability of different textile fabrics using micro-scale combustion calorimetry. Polym. Degrad. Stab. 2010, 95, 108–115. [Google Scholar] [CrossRef]
- Brauman, S.K.; Swidler, R.; Trescony, P.V.; Brolly, A.S. N-sulfonyl phosphoramidate fire retardants in poly(ethylene terephthalate). J. Fire Retard. Chem. 1980, 7, 15–26. [Google Scholar]
- Chen, L.; Song, L.; Lv, P.; Jie, G.; Tai, Q.; Xing, W.; Hu, Y. A new intumescent flame retardant containing phosphorus and nitrogen: Preparation, thermal properties and application to UV curable coating. Prog. Org. Coat. 2011, 70, 59–66. [Google Scholar] [CrossRef]
- Gaan, S.; Mauclaire, L.; Rupper, P.; Salimova, V.; Tran, T.-T.; Heuberger, M. Thermal degradation of cellulose acetate in presence of bis-phosphoramidates. J. Anal. Appl. Pyrolysis 2011, 90, 33–41. [Google Scholar] [CrossRef]
- Neisius, M.; Liang, S.; Mispreuve, H.; Gaan, S. Phosphoramidate-containing flame-retardant flexible polyurethane foams. Ind. Eng. Chem. Res. 2013, 52, 9752–9762. [Google Scholar] [CrossRef]
- Schmid, H.; Gaan, S. Aromatic Bis-phosphoramidate Additives as Flame Retardants for Polymers. EP2481744A1, 1 August 2012. [Google Scholar]
- Zhao, W.; Li, B.; Xu, M.; Yang, K.; Lin, L. Novel intumescent flame retardants: Synthesis and application in polycarbonate. Fire Mater. 2013, 37, 530–546. [Google Scholar] [CrossRef]
- Pandya, H.B.; Bhagwat, M.M. Mechanistic aspects of phosphorus-nitrogen synergism in cotton flame retardancy. Text. Res. J. 1981, 51, 5–8. [Google Scholar] [CrossRef]
- Liang, S.; Neisius, M.; Mispreuve, H.; Naescher, R.; Gaan, S. Flame retardancy and thermal decomposition of flexible polyurethane foams: Structural influence of organophosphorus compounds. Polym. Degrad. Stab. 2012, 97, 2428–2440. [Google Scholar] [CrossRef]




| Material and description | P-Cell (wt %) | Cot-Cell (wt %) | |
|---|---|---|---|
| Brown fibers | White fibers | ||
| Composition of the textile | 15 | 85 | 100 |
| Lignin | 30 | 0 | 0 |
| Hemicellulose | 16 | 0 | 0 |
| Cellulose | 54 | 100 | 100 |
| Sample | After-flame (s) | After-glow (s) | Burn length |
|---|---|---|---|
| Cot-Cell | 36.0 (± 1.41) | 163.3 (± 15.2) | burns completely |
| P-Cell | 18.6 (± 2.0) | 263.3 (± 4.0) | burns completely |
| Sample | TdPeak °C | % Residue at 800 °C |
|---|---|---|
| Cot-Cell | 363.7 | 3.4 |
| P-Cell | 360.07 | 13.00 |
| P-Cell Brown Fiber | 348.5 | 15.57 |
| P-Cell-White Fiber | 365.5 | 4.65 |
| Sample | THR (kJ/g) | Tmax (°C) | Qmax (W/g) | % Residue |
|---|---|---|---|---|
| Cot-Cell | 11.8 ± 0.5 | 370.3 ± 0.9 | 263.5 ± 5.9 | 5.6 ± 0.8 |
| P-Cell | 11.9 ± 0.1 | 361.6 ± 10.4 | 266.3 ± 1.9 | 5.8 ± 0.6 |
| P-Cell White Fibers | 12.1 ± 0.6 | 366.2 ± 2.0 | 266.3 ± 1.8 | 7.6 ± 1.9 |
| P-Cell-Brown Fibers | 8.5 ± 0.2 | 359.5 ± 1.9 | 151.8 ± 4.1 | 11.5 ± 4.3 |
| Cellulose sample | FR type | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AA-DEP | AA-DMP | BA-DEP | BA-DMP | DA-DEP | DA-DMP | EDA-DEP | EDA-DMP | PA-DEP | PA-DMP | |
| Cot-Cell | 2.1 | 1.7 | 1.9 | 2.0 | 1.8 | 1.8 | 2.2 | 1.7 | 1.7 | 2.0 |
| P-Cell | 1.8 | 2.1 | 1.9 | 2.0 | 1.9 | 1.7 | 1.9 | 1.7 | 2.0 | 2.2 |
| FR | Cellulose type | TdPeak °C | % Residue |
|---|---|---|---|
| Untreated | Cot-Cell | 369 | 3.4 |
| P-Cell | 366 | 13.0 | |
| AA-DEP | Cot-Cell | 321 | 22.2 |
| P-Cell | 296 | 27.8 | |
| AA-DMP | Cot-Cell | 299 | 28.9 |
| P-Cell | 297 | 31.0 | |
| BA-DEP | Cot-Cell | 315 | 14.1 |
| P-Cell | 300 | 24.6 | |
| BA-DMP | Cot-Cell | 292 | 23.5 |
| P-Cell | 292 | 26.5 | |
| DA-DEP | Cot-Cell | 319 | 14.7 |
| P-Cell | 319 | 23.7 | |
| DA-DMP | Cot-Cell | 317 | 15.7 |
| P-Cell | 318 | 26.3 | |
| EDA-DEP | Cot-Cell | 293 | 27.1 |
| P-Cell | 290 | 30.4 | |
| EDA-DMP | Cot-Cell | 285 | 34.3 |
| P-Cell | 292 | 33.5 | |
| PA-DEP | Cot-Cell | 298 | 23.0 |
| P-Cell | 295 | 25.4 | |
| PA-DMP | Cot-Cell | 289 | 31.5 |
| P-Cell | 290 | 32.5 |
| Sample | THR (kJ/g) | Tinit (°C) | Tmax (°C) | Qint (W/g) | Qmax (W/g) | % Residue |
|---|---|---|---|---|---|---|
| Untreated | 11.0 ± 0.2 | 370.3 ± 0.5 | 256.3 ± 4.8 | 5.8 ± 0.6 | ||
| AA-DEP | 6.5 ± 0.2 | 137.6 ± 0.6 | 319.8 ± 1.3 | 31.7 ± 4.3 | 246.5 ± 6.9 | 12.54 ± 1.6 |
| AA-DMP | 4.2 ± 0.5 | 136.0 ± 7.9 | 291.4 ± 0.7 | 8.5 ± 2.4 | 187.9 ± 2.8 | 25.9 ± 0.5 |
| BA-DEP | 10.9 ± 0.2 | 197.4 ± 2.0 | 314.0 ± 1.3 | 70.8 ± 3.2 | 227.1 ± 3.8 | 12.2 ± 0.1 |
| BA-DMP | 9.7 ± 0.5 | 188.7 ± 1.0 | 289.0 ± 0.6 | 48.0 ± 1.4 | 170.3 ± 6.3 | 21.2 ± 0.9 |
| DA-DEP | 9.0 ± 0.6 | 318.4 ± 4.1 | 262.3 ± 7.5 | 14.7 ± 3.5 | ||
| DA-DMP | 8.6 ± 1.2 | 325.3 ± 7.3 | 250.6 ± 3.3 | 15.5 ± 3.7 | ||
| EDA-DEP | 4.5 ± 0.5 | 292.2 ± 4.8 | 157.5 ± 15.0 | 26.5 ± 0.9 | ||
| EDA-DMP | 2.8 ± 0.3 | 277.0 ± 1.4 | 126.1 ± 4.0 | 27.5 ± 0.9 | ||
| PA-DEP | 7.2 ± 0.1 | 304.0 ± 3.1 | 207.2 ± 2.9 | 16.7 ± 1.0 | ||
| PA-DMP | 4.5 ± 0.1 | 283.5 ± 2.4 | 178.4 ± 5.8 | 25.9 ± 1.7 |
| Sample | THR (kJ/g) | Tinit (°C) | Tmax (°C) | Qint (W/g) | Qmax (W/g) | % Residue at 900 °C |
|---|---|---|---|---|---|---|
| Untreated | 11.9 ± 0.1 | 361.6 ± 1.8 | 266.3 ± 9.9 | 5.6 ± 0.8 | ||
| AA-DEP | 4.9 ± 0.3 | 289.2 ± 1.7 | 152.8 ± 7.0 | 23.8 ± 0.4 | ||
| AA-DMP | 3.8 ± 0.2 | 287.5 ± 2.1 | 146.2 ± 2.2 | 30.0 ± 0.8 | ||
| BA-DEP | 9.3 ± 0.7 | 183.5 ± 1.9 | 289.7 ± 1.8 | 32.5 ± 1.0 | 169.6 ± 4.3 | 18.5 ± 0.4 |
| BA-DMP | 8.6 ± 0.8 | 178.9 ± 1.4 | 283.2 ± 0.7 | 33.6 ± 1.2 | 153.6 ± 5.9 | 27.1 ± 0.4 |
| DA-DEP | 9.9 ± 0.7 | 329.8 ± 0.9 | 177.7 ± 0.8 | 12.3 ± 0.5 | ||
| DA-DMP | 9.0 ± 0.5 | 331.5 ± 1.4 | 155.6 ± 3.8 | 14.1 ± 1.5 | ||
| EDA-DEP | 3.6 ± 0.5 | 285.0 ± 3.3 | 97.6 ± 5.2 | 31.2 ± 0.4 | ||
| EDA-DMP | 2.8 ± 0.5 | 283.2 ± 0.5 | 124.6 ± 6.1 | 32.1 ± 1.2 | ||
| PA-DEP | 6.1 ± 0.1 | 290.8 ± 2.0 | 176.3 ± 2.0 | 23.2 ± 0.4 | ||
| PA-DMP | 3.7 ± 0.3 | 278.5 ± 1.5 | 133.7 ± 2.3 | 28.4 ± 0.5 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmeia, K.A.; Jovic, M.; Ragaisiene, A.; Rukuiziene, Z.; Milasius, R.; Mikucioniene, D.; Gaan, S. Flammability of Cellulose-Based Fibers and the Effect of Structure of Phosphorus Compounds on Their Flame Retardancy. Polymers 2016, 8, 293. https://doi.org/10.3390/polym8080293
Salmeia KA, Jovic M, Ragaisiene A, Rukuiziene Z, Milasius R, Mikucioniene D, Gaan S. Flammability of Cellulose-Based Fibers and the Effect of Structure of Phosphorus Compounds on Their Flame Retardancy. Polymers. 2016; 8(8):293. https://doi.org/10.3390/polym8080293
Chicago/Turabian StyleSalmeia, Khalifah A., Milijana Jovic, Audrone Ragaisiene, Zaneta Rukuiziene, Rimvydas Milasius, Daiva Mikucioniene, and Sabyasachi Gaan. 2016. "Flammability of Cellulose-Based Fibers and the Effect of Structure of Phosphorus Compounds on Their Flame Retardancy" Polymers 8, no. 8: 293. https://doi.org/10.3390/polym8080293