Synthesis of Self-Gravity Settling Faceted-Anatase TiO2 with Dominant {010} Facets for the Photocatalytic Degradation of Acetaminophen and Study of the Type of Generated Oxygen Vacancy in Faceted-TiO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of T-{010}
2.2. Experimental Procedure and Sample Preparation
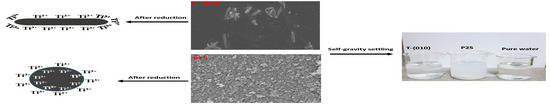
2.3. Sedimentation of P25 and T-{010}
2.4. Preparation of Colored T-{010} (BT-{010}) and P25 (BP25) by NaBH4 Reduction
2.5. Instruments
3. Results and Discussions
3.1. Characterization
3.2. Photocatalytic Degradation
3.3. Photocatalytic Activity of BT-{010} and BP25
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choquette-Labbé, M.; Shewa, W.A.; Lalman, J.A.; Shanmugam, S.R. Photocatalytic degradation of phenol and phenol derivatives using a nano-TiO2 Catalyst: Integrating quantitative and qualitative factors using response surface methodology. Water 2014, 6, 1785–1806. [Google Scholar] [CrossRef]
- Mingquan, W.; Fangshu, Q.; Ruibao, J.; Shaohua, S.; Guibai, L.; Heng, L. Preliminary Study on the Removal of Steroidal Estrogens Using TiO2-Doped PVDF Ultrafiltration Membranes. Water 2016, 8, 134. [Google Scholar]
- Liu, G.; Yu, J.C.; Lu, G.Q.; Cheng, H.-M. Crystal facet engineering of semiconductor photocatalysts: Motivations, advances and unique properties. Chem. Commun. 2011, 47, 6763–6766. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wu, X.; Wang, L.; Liu, G.; Lu, G.Q.; Cheng, H.-M. Synthesis of anatase TiO2 rods with dominant reactive {010} facets for the photoreduction of CO2 to CH4 and use in dye-sensitized solar cells. Chem. Commun. 2011, 47, 8361–8365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Han, Y.; Liu, P.; Yao, X.; Zhao, H. A selective etching phenomenon on {001} faceted anatase titanium dioxide single crystal surfaces by hydrofluoric acid. Chem. Commun. 2011, 47, 2829–2833. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Qin, Y.; Cao, Q.; Hu, B.; Huang, Z.; Ye, L.; Tang, X. Novel photocatalytic antibacterial activity of TiO2 microspheres exposing 100% reactive {111} facets. Chem. Commun. 2011, 47, 12628–12632. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, J.; Wang, Y.; Zhu, F.; Shi, W.; Wan, F.; Xu, D. A facile synthesis of anatase TiO2 nanosheets-based hierarchical spheres with over 90% {001} facets for dye-sensitized solar cells. Chem. Commun. 2011, 47, 1809–1811. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, M.; Vittadini, A.; Selloni, A. Erratum: Structure and energetics of stoichiometric TiO2 anatase surfaces. Phys. Rev. B 2002, 65, 119901–119907. [Google Scholar] [CrossRef]
- Wen, C.Z.; Zhou, J.Z.; Jiang, H.B.; Hu, Q.H.; Qiao, S.Z.; Yang, H.G. Synthesis of micro-sized titanium dioxide nanosheets wholly exposed with high-energy {001} and {100} facets. Chem. Commun. 2011, 47, 4400–4403. [Google Scholar] [CrossRef] [PubMed]
- Parıltı, N.B.; Akten, D. Application of Box–Wilson experimental design method for the solar photocatalytic degradation of textile dyestuff with Fe(III)/H2O2/solar UV process. Desalination 2010, 260, 193–198. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Hushmandrad, S. Solar photodecolorization of methylene blue by CuO/X zeolite as a heterogeneous catalyst. Appl. Catal. A Gen. 2010, 388, 149–159. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Peter, Y.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Li, M.; Gao, C.; Zhang, G.; Zhang, P.; Wang, Y.; Chen, L.; Xie, E. Preparation of black TiO2 by hydrogen plasma assisted chemical vapor deposition and its photocatalytic activity. Appl. Catal. B Environ. 2014, 148, 339–343. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Cao, J.; Zhang, Y.; Liu, L.; Xu, H.; Ye, J. Reduced TiO2 nanotube arrays for photoelectrochemical water splitting. J. Mater. Chem. A 2013, 1, 5766–5774. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Guan, Z.; Li, Q.; He, C.; Yang, J. Synergistic effect of surface and bulk single-electron- trapped oxygen vacancy of TiO2 in the photocatalytic reduction of CO2. Appl. Catal. B Environ. 2017, 206, 300–307. [Google Scholar] [CrossRef]
- Yang, H.G.; Liu, G.; Qiao, S.Z.; Sun, C.H.; Jin, Y.G.; Smith, S.C.; Zou, J.; Cheng, H.M.; Lu, G.Q. (Max) Solvothermal Synthesis and Photoreactivity of Anatase TiO2 Nanosheets with Dominant {001} Facets. J. Am. Chem. Soc. 2009, 131, 4078–4083. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Du, P.; Cai, Z.; Wang, T.; Fu, J.; Liu, W. Photocatalysis of bisphenol A by an easy-settling titania/titanate composite: Effects of water chemistry factors, degradation pathway and theoretical calculation. Environ. Pollut. 2018, 232, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Mao, J.; Liu, J.; Jiang, Z.; Peng, T.; Zan, L. Synthesis of anatase TiO2 nanocrystals with {101}, {001} or {010} single facets of 90% level exposure and liquid phase photocatalytic reduction and oxidation activity orders. J. Mater. Chem. A 2013, 1, 10532–10537. [Google Scholar] [CrossRef]
- Pan, J.; Liu, G.; Lu, G.Q.M.; Cheng, H.-M. On the True Photoreactivity Order of {001}, {010}, and {101} Facets of Anatase TiO2 Crystals. Angew. Chem. Int. Ed. 2011, 50, 2133–2137. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Burda, C. The Electronic Origin of the Visible-Light Absorption Properties of C-, N- and S-Doped TiO2 Nanomaterials. Am. Chem. Soc. 2008, 130, 5018–5019. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Koenigsmann, C.; Ding, W.; Rudshteyn, B.; Yang, K.R.; Regan, K.P.; Konezny, S.J.; Batista, V.S.; Brudvig, G.W.; Schmuttenmaer, C.A.; Kim, J. Facet-Dependent Photoelectrochemical Performance of TiO2 Nanostructures: An Experimental and Computational Study. J. Am. Chem. Soc. 2015, 137, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, L.S.; Sczancoski, J.C.; Varela, J.A.; Longo, E.; Andrés, J.; Rout, S.K. Photoluminescence Properties of Nanocrystals. J. Nanomater. 2012, 2012, 1–2. [Google Scholar] [CrossRef]
- Zhu, Q.; Peng, Y.; Lin, L.; Fan, C.-M.; Gao, G.-Q.; Wang, R.-X.; Xu, A.-W. Stable blue TiO2−x nanoparticles for efficient visible light photocatalysts. J. Mater. Chem. A 2014, 2, 4429–4433. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Y.; Zhao, H.; Chen, J.; Cheng, J.; Yang, K.; Li, Y. Engineering Coexposed {001} and {101} Facets in Oxygen-Deficient TiO2 Nanocrystals for Enhanced CO2 Photoreduction under Visible Light. ACS Catal. 2016, 6, 1097–1108. [Google Scholar] [CrossRef]
- Masudy-Panah, S.; Siavash Moakhar, R.; Chua, C.S.; Tan, H.R.; Wong, T.I.; Chi, D.; Dalapati, G.K. Nanocrystal Engineering of Sputter-Grown CuO Photocathode for Visible-Light-Driven Electrochemical Water Splitting. ACS Appl. Mater. Interfaces 2016, 8, 1206–1213. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katal, R.; Masudy Panah, S.; Zarinejad, M.; Salehi, M.; Jiangyong, H. Synthesis of Self-Gravity Settling Faceted-Anatase TiO2 with Dominant {010} Facets for the Photocatalytic Degradation of Acetaminophen and Study of the Type of Generated Oxygen Vacancy in Faceted-TiO2. Water 2018, 10, 1462. https://doi.org/10.3390/w10101462
Katal R, Masudy Panah S, Zarinejad M, Salehi M, Jiangyong H. Synthesis of Self-Gravity Settling Faceted-Anatase TiO2 with Dominant {010} Facets for the Photocatalytic Degradation of Acetaminophen and Study of the Type of Generated Oxygen Vacancy in Faceted-TiO2. Water. 2018; 10(10):1462. https://doi.org/10.3390/w10101462
Chicago/Turabian StyleKatal, Reza, Saeid Masudy Panah, Mehrdad Zarinejad, Mojtaba Salehi, and Hu Jiangyong. 2018. "Synthesis of Self-Gravity Settling Faceted-Anatase TiO2 with Dominant {010} Facets for the Photocatalytic Degradation of Acetaminophen and Study of the Type of Generated Oxygen Vacancy in Faceted-TiO2" Water 10, no. 10: 1462. https://doi.org/10.3390/w10101462