Abstract
We prepared crystals of (C6H5CnH2nNH3)2PbBr4 (n = 2, 3, 4), and evaluated their photoluminescence and scintillation characteristics. According to the photoluminescence spectra under 340 nm excitation light, a photoluminescence peak at around 410 nm due to free exciton emissions appeared in (C6H5C2H4NH3)2PbBr4 (2PEA), (C6H5C3H6NH3)2PbBr4 (3PPA) and (C6H5C4H8NH3)2PbBr4 (4PBA). The quantum efficiencies of 2PEA, 3PPA and 4PBA were 0.24, 0.01 and 0.04, respectively. In scintillation, a sharp scintillation peak originating from the exciton emissions was detected at approximately 436 nm for 2PEA, 459 nm for 3PPA and 442 nm for 4PBA. In addition, the scintillation light yield of 4PBA under 59.5 keV gamma-ray irradiation from 241Am was determined to be approximately 2200 photons MeV−1 in comparison with the yield of 2PEA (14 000 photons MeV−1), whereas the yield of 3PPA could not be determined due to its low scintillation intensity.
Export citation and abstract BibTeX RIS
1. Introduction
Scintillators are a kind of fluorescent material for the measurement of high-energy ionizing radiation. They have the ability to absorb ionizing radiation, and immediately convert into low-energy photons that can be collected by a photodetector, such as a photomultiplier tube with the aim of measuring high-energy ionizing radiation. 1–3) Scintillators have been used for decades in many applications, such as security and medical imaging. 4–6) To date, a wide variety of scintillators, such as inorganic materials, organic materials and organic–inorganic hybrid materials have been developed. In particular, inorganic scintillators have been utilized in various applications. 7) For example, CdWO4 is a well-known inorganic scintillator used for X-ray computed tomography. 8,9) It shows an emission at 480 nm originating from charge-transfer transitions between oxygen (O2−) and tungsten (W6+) ions, and the scintillation light yield is about 15 000 photons MeV−1. 10–12) Furthermore, Ce-doped Gd2SiO5 (GSO:Ce) is also a well-known inorganic scintillator, which displays a moderate scintillation light yield of approximately 10000 photons MeV−1 and a fast decay time of 48 ns due to the electronic 5d-4f transitions of Ce3+. 13,14) To date, it has been used for several applications, such as positron emission tomography and well logging. 14–16) In addition, some organic materials and organic–inorganic hybrid materials have been developed as organic single-crystal scintillators, liquid scintillators and plastic scintillators for use in several applications, such as neutron and gamma-ray pulse shape discrimination, and spectroscopies at a high-energy accelerator. 17–19)
In recent years, organic–inorganic perovskite-type compounds with a chemical composition of (RNH3)2PbX4 (R: hydrocarbon, X: halogen) have attracted research attention as a fast-response scintillator, which is characterized by a high scintillation light yield and a fast decay time on the order of nanoseconds. 20) They have self-organized laminated structure in which inorganic layers are sandwiched by organic layers, and they are recognized as a quantum well structure due to the large energy-gap difference between inorganic and organic layers. 13,21–25) To date, these compounds have been reported to exhibit efficient exciton emissions with a fast decay time on the order of a few nanoseconds due to the quantum confinement effect. 26) In particular, it has been demonstrated that (C6H5C2H4NH3)2PbBr4 (2PEA) shows a fast decay time constant of 11 ns and a high light yield of 14 000 photons MeV−1. 13) Due to their unique exciton properties, the organic–inorganic layered perovskite-type compounds with the composition of (RNH3)2PbX4 should be promising candidates to be used as fast-response scintillators.
In previous works, the photoluminescence (PL) and scintillation characteristics of (C6H5CH2NH3)2PbBr4 (Ben) and 2PEA have been investigated, and it has been found that the side chain length of the benzene ring in the organic layer has a significant effect on PL and scintillation properties. According to a previous study, the quantum efficiency (QE) values in PL are 0.04 for Ben and 0.22 for 2PEA, and the scintillation light yield of Ben is 0.24 times that of 2PEA. 23) To investigate the cause of different luminescent intensities, single-crystal structure analysis of Ben and 2PEA has been performed, and the analysis has demonstrated that 2PEA has a larger structural distortion in the inorganic layer than Ben, and the structural distortion leads to an increase in the effective mass of excitons and a decrease in the Bohr radius of excitons, resulting in a higher QE value and scintillation light yield. 22,23,27) To further investigate the effect of the side chain length on the PL and scintillation properties, we fabricated (C6H5C3H6NH3)2PbBr4 (3PPA) and (C6H5C4H8NH3)2PbBr4 (4PBA) crystals having a longer side chain than 2PEA and Ben. The crystals were fabricated using a temperature gradient method. After fabrication, we evaluated the PL and scintillation properties of 3PPA and 4PBA, and compared them with 2PEA.
2. Experimental methods
We used C6H5C2H4NH2 (2-phenylethylamine), C6H5C3H6NH2 (3-phenylpropylamine) and C6H5C4H8NH2 (4-phenylbutylamine) as organic precursors. The organic precursor was mixed with hydrobromic acid, and the mixed aqueous solution that contained the precursor and hydrogen bromide in a molar ratio of 1:1 was stirred for 30 min. After evaporating the solvent, C6H5Cn H2n NH3Br (n = 2, 3, 4) polycrystalline powder was formed. Then, the powder and lead bromide were stirred for 2 h in a molar ratio of 2:1 in N,N-dimethylformamide (DMF). (C6H5C2H4NH3)2PbBr4 (2PEA), (C6H5C3H6NH3)2PbBr4 (3PPA) and (C6H5C4H8NH3)2PbBr4 (4PBA) polycrystalline powders were then obtained by evaporating the DMF. Moreover, 2PEA, 3PPA and 4PBA crystals were fabricated as follows. Each powder was dissolved and stirred in DMF (good solvent) in a screw tube bottle, and nitromethane (poor solvent) was dropped into the solution until the powders precipitated. Next, the precipitated powder in the screw tube bottle was dissolved at 90 °C, and the screw tube bottle was stored in a synthesizer (Chemi Chemi-300, Shibata) at 90 °C. Finally, 2PEA, 3PPA and 4PBA crystals with a thickness of approximately 0.2 mm were obtained by gradually cooling at room temperature at a rate of 3.5 °C h−1. Photographs of the 2PEA, 3PPA and 4PBA crystals are presented in Fig. 1.
Fig. 1. (Color online) Photographs of 2PEA, 3PPA and 4PBA crystals.
Download figure:
Standard image High-resolution imageX-ray diffraction (XRD) patterns of the 2PEA, 3PPA and 4PBA crystals were measured using a diffractometer (RINT2000, Rigaku). The PL excitation and emission maps, and QE values were measured using a spectrometer (Quantaurus-QY C11347, Hamamatsu), while the PL spectra were recorded using a fluorescent spectrometer (RF-6000, Shimazu). PL decay curves were obtained by using a spectrometer (Quantaurus-tau C11367, Hamamatsu). The scintillation spectra were measured under X-ray irradiation from an X-ray generator (XRB80N100, Spellman). Scintillation decay curves were recorded by using our original afterglow system. 28) In addition, the pulse-height spectra were measured in order to investigate the scintillation light yield under 241Am gamma-ray (59.5 keV) irradiation. Details of the pulse-height spectra are provided in Ref. 13.
3. Results and discussion
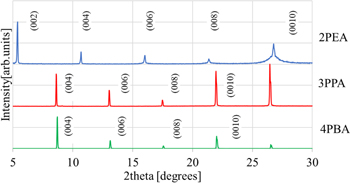
Figure 2 represents XRD patterns of the 2PEA, 3PPA and 4PBA crystals. As displayed in Fig. 2, several diffraction patterns appeared in the range from 5°–30° for 2PEA, 3PPA and 4PBA crystals. All diffraction peaks should be ascribed to (0 0 2 l) (l = 1, 2, 3, 4, 5) from the previous study on 2PEA crystal, indicating that a quantum well structure was formed in the 2PEA, 3PPA and 4PBA crystals. 13) In addition, the d004 values were calculated to be 8.3 Å for 2PEA, 10.3 Å for 3PPA and 10.2 Å for 4PBA. In general, the values are expected to increase with increasing the side chain length on the benzene ring since the organic amines are oriented perpendicular to the inorganic layer in the case of the compounds with a multiple quantum well structure. 22) The reason the 3PPA and 4PBA crystals showed almost the same values might be that the organic amines in the 4PEA crystal might be slightly tilted parallel to the inorganic layer, compared with the 3PPA crystal.
Fig. 2. (Color online) XRD patterns of the 2PEA, 3PPA and 4PBA crystals.
Download figure:
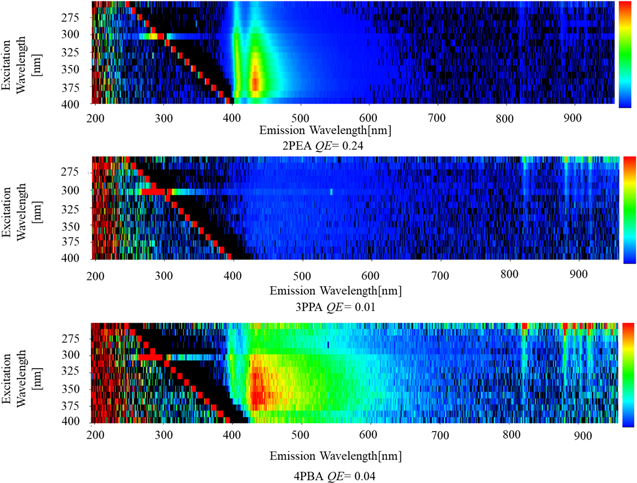
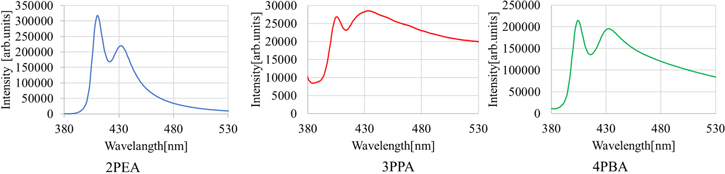
Standard image High-resolution imageFigure 3 presents the PL excitation and emission maps of the 2PEA, 3PPA and 4PBA crystals. The 2PEA and 4PBA crystals showed emission signals clearly under excitation wavelength range from 300–380 nm, while no clear signal from the 3PPA crystals was detected. PL spectra of the 2PEA, 3PPA and 4PBA crystals under 340 nm excitation light were displayed in Fig. 4. An emission peak was observed at 411 nm for the 2PEA crystals, 406 nm for the 3PPA crystals and 405 nm for the 4PBA crystals. These peaks originated from a free exciton recombination in the inorganic layer. 13,29,30) Moreover, a broad emission peaking at 432, 434 and 434 nm was probably due to bound excitons at defects that were detected in the 2PEA, 3PPA and 4PBA crystals, respectively. 13,30) The peaks for the 3PPA and 4PBA crystals were broader than that of the 2PEA crystal, which suggests that many kinds of defects with deeper levels were formed in the 3PPA and 4PBA crystals. In addition, the QE values were recorded when excited by 340 nm excitation light, and the QE values for the 2PEA, 3PPA and 4PBA crystals were calculated to be 0.24, 0.01 and 0.04, respectively. The QE values were not improved by increasing the side chain length of the benzene ring in the organic layer. The observed low QE values suggest that the further structural distortions in the inorganic layer might not be induced by increasing the side chain length in the 3PPA and 4PBA crystals compared with the 2PEA crystal. In addition, observation of the broader peak for the 3PPA and 4PBA crystals might be related to the low QE values since many defects associated with non-radiative decay loss might also be formed in the 3PPA and 4PBA crystals, compared with the 2PEA crystal.
Fig. 3. (Color online) PL excitation and emission maps of the 2PEA, 3PPA and 4PBA crystals.
Download figure:
Standard image High-resolution imageFig. 4. (Color online) PL spectra of the 2PEA, 3PPA and 4PBA crystals.
Download figure:
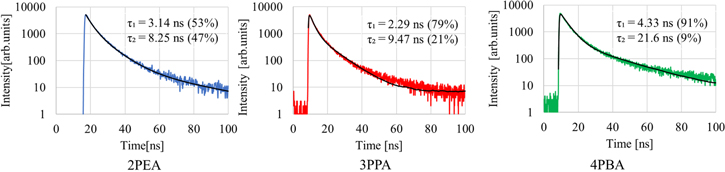
Standard image High-resolution imageFigure 5 presents PL decay curves of the 2PEA, 3PPA and 4PBA crystals at a log scale. The curves were recorded under 340 nm excitation light, monitored at the exciton emission wavelengths of each crystal. Two components of the decay curves were observed in the 2PEA, 3PPA and 4PBA crystals. Based on the assumption of the decay time profiles with the sum of two exponential decay functions, PL decay time constants of the first component were estimated to be 3.14 ns (2PEA), 2.29 ns (3PPA) and 4.33 ns (4PBA), while the constants of the second component were 8.25 ns (2PEA), 9.47 ns (3PPA) and 21.6 ns (4PBA). Compared to past study on 2PEA crystals, it is presumed that the origins of the first and second components were free excitons, and bound excitons trapped at unknown defects, respectively. 13,21)
Fig. 5. (Color online) PL decay curves of the 2PEA, 3PPA and 4PBA crystals.
Download figure:
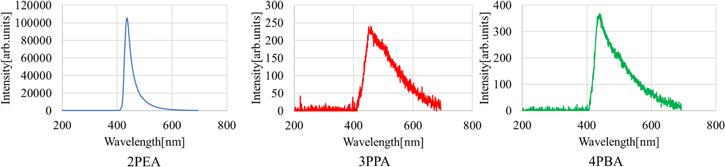
Standard image High-resolution imageFigure 6 presents X-ray-induced scintillation spectra of the 2PEA, 3PPA and 4PBA crystals. An emission peak, probably due to bound excitons at defects, was observed at approximately 436 nm for 2PEA crystals, 459 nm for 3PPA crystals and 442 nm for 4PBA crystals. The peak top wavelengths of the 2PEA, 3PPA and 4PBA crystals were found to shift to the longer wavelength side in scintillation. The redshift should be due to the different measurement methods. In PL, the photons emitted from the surface of the samples were recorded, whereas only the photons transmitted through the samples were collected. Therefore, the emission at around 410 nm might be absorbed by the sample itself because of a low Stokes shift. 31)
Fig. 6. (Color online) X-ray-induced scintillation spectra of the 2PEA, 3PPA and 4PBA crystals.
Download figure:
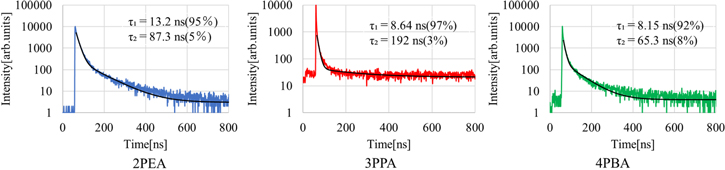
Standard image High-resolution imageFigure 7 presents the X-ray-induced scintillation decay curves of the 2PEA, 3PPA and 4PBA crystals at a log scale. Two components of the scintillation decay curves were observed in the 2PEA, 3PPA and 4PBA crystals. The decay curves were approximated by the sum of two exponential decay functions, and the derived constants of the faster component were 13.2 ns (2PEA), 8.64 ns (3PPA) and 8.15 ns (4PBA), whereas the constants of the slower component were 87.3 ns (2PEA), 192 ns (3PPA) and 65.3 ns (4PBA). The faster component was thought to originate from the free excitons in the inorganic layer while the slower component might derive from bound excitons trapped at unknown defects. 13,32) The scintillation decay time constants for the 2PEA, 3PPA and 4PBA crystals were found to be higher than the PL decay time constants. It is known that the scintillation phenomenon is composed of three different processes: (1) excitation and relaxation of charge carriers, (2) energy transfer to a luminescent center, (3) emission at the final luminescent center, and the third stage is supposed to be the same as the third process. 33,34) The scintillation decay time constant is sometimes greater than the PL decay time constant because of the additional first and second processes.
Fig. 7. (Color online) X-ray-induced scintillation decay curves of the 2PEA, 3PPA and 4PBA crystals.
Download figure:
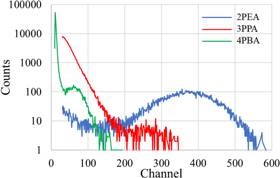
Standard image High-resolution imageFigure 8 presents the pulse-height spectra of the 2PEA, 3PPA and 4PBA crystals under 241Am gamma-ray (59.5 keV) excitation. A full-energy peak was detected in the 2PEA and 4PBA crystals, and the channel numbers of the 2PEA and 4PBA crystals were 372 and 59, respectively. On the other hand, a weak signal of the 3PPA crystal was observed at around 250 channel. The origin of the signal for the 3PPA crystal should be a peak pile-up because of its low QE value. Thus, its scintillation light yield could not be determined in this study. Previous studies have reported that the yield of 2PEA was determined to be 14000 photons MeV−1. 13) The yield of 4PBA was about 2200 photons MeV−1 (4PBA), assuming that the yield is proportional to the channel number. Based on a theoretical model, the scintillation light yield (LY) can be expressed by the following equation.
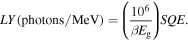
Here, the energy transfer efficiency to the final luminescence center is S, the quantum efficiency is QE, the energy-gap energy is  and the constant dependent on materials is β.
33,34) In this study, we found that the scintillation light yields of the 2PEA, 3PPA and 4PBA crystals were well correlated with QE values. Thus, the difference in scintillation light yields among the 2PEA, 3PPA and 4PBA crystals should originate from mainly QE values, which are reported to be mainly dependent on exciton properties. In this work, the scintillation light yields were not improved by increasing the side chain length of the benzene ring. However, the scintillation decay time constants decreased by increasing the side chain length. Moreover, the clear correlation between scintillation light yields and QE values was found to be observed.
and the constant dependent on materials is β.
33,34) In this study, we found that the scintillation light yields of the 2PEA, 3PPA and 4PBA crystals were well correlated with QE values. Thus, the difference in scintillation light yields among the 2PEA, 3PPA and 4PBA crystals should originate from mainly QE values, which are reported to be mainly dependent on exciton properties. In this work, the scintillation light yields were not improved by increasing the side chain length of the benzene ring. However, the scintillation decay time constants decreased by increasing the side chain length. Moreover, the clear correlation between scintillation light yields and QE values was found to be observed.
Fig. 8. (Color online) Pulse-height spectra of the 2PEA, 3PPA and 4PBA crystals under 241Am gamma-ray (59.5 keV) excitation.
Download figure:
Standard image High-resolution image4. Conclusion
(C6H5Cn H2n NH3)2PbBr4 {n = 2, 3, 4} crystals were synthesized to investigate the side chain length of the PL and scintillation properties. All crystals showed exciton emissions with a short decay time constant of a few nanoseconds in PL and scintillation. Moreover, the scintillation light yields of (C6H5C3H6NH3)2PbBr4 and (C6H5C4H8NH3)2PbBr4 under gamma-ray irradiation were lower than the yield of (C6H5C2H4NH3)2PbBr4. Throughout this research, the PL and scintillation intensities were not improved by increasing the side chain length. However, a clear relationship between QE values and scintillation light yields was observed.