Abstract
Quantum-confined cesium lead tribromide perovskite quantum dots (PeQDs) are attractive photonic sources for versatile optoelectronic devices and thus the synthetic routes have been explored via various techniques. However, the lack of a general method to fabricate the quantum-confined PeQD with remarkable stability at colloidal state has been one of the major obstacles to device applications. Herein, we propose a simple and robust ligand-assisted reprecipitation method associated with didodecyl dimethyl ammonium bromide for quantum-confined PeQDs with remarkable stability at colloidal state. This method will shed a light on the field of colloidal PeQDs synthesis for the next generation of photonic sources.
Export citation and abstract BibTeX RIS
Cesium lead trihalide (CsPbX3, X = Cl, Br, and I) Perovskite Quantum Dots (PeQDs) are promising next-generation semiconductor and photoconversion materials for various devices such as solar cells, 1,2) light-emitting diodes, 3–5) lasers, 6) and enhancement films. 7,8) Their unique and excellent optical properties are not only large absorption coefficient and narrow-band emission line width, 9,10) but only originated from the quantum confinement resulting in the strong exciton binding energy and high photoluminescent quantum yields (PLQYs). 11–13) Especially, the quantum confinement effect is of importance to the multiple exciton generation and tunability of the absorption and emission wavelengths, which play important roles in photonic devices. 10,14) However, the fabrication method for quantum-confined PeQD has not been established, which has been one of the major obstacles to their practical applications. To this end, the development of a simple synthetic root for quantum-confined PeQDs is expected to hold high potential to tackle this issue.
Various synthetic routes, such as hot injection (HI) synthesis, 3,4,10,13,15) and exfoliation approaches associated with ultrasonication, 16) microwave, 17) and/or capping ligands, 18) and ligand-assisted reprecipitation (LARP) methods, 19–22) have been explored to extend the color tunability of single-halide PeQDs. HI is the most widely recognized method to realize fine quantum confinement in nanometer-sized PeQDs. 15) However, this method is highly demanding as such that has to be carried out at high temperature over 140 °C and under an oxygen-free atmosphere. 10) The exfoliation methods offer mild fabrication conditions, but the precise size-control of the colloidal QDs remains challenging. The LARP has been recently proposed as a promising alternative because it can provide fine colloidal PeQDs with high luminescence quantum yield despite its simple protocol. 19,21)
In the LARP, colloidal PeQDs are prepared through rapid injection of precursors dissolved in a good solvent (typically polar solvent) into a poor solvent (typically non-polar solvent), leading to rapid nucleation followed by crystal growth due to the reprecipitation effect. The nucleation and growth rate obviously can be controlled by optimizing reaction temperature from cryogenic 20) to room temperature along with appropriate choices of capping ligands. (e.g. octanoic acid, oleic acid, octylamine, oleylamine) A mixture of carboxylic acid and alkyl amine is often used as capping ligands to control crystal growth. However, the proton exchange between these ligands could lead to the detachment of the ligands. 23,24) Because the reaction mixture contains a small portion of the polar solvent from the precursor solution, the crystal growth of PeQDs after the ligand detachment undergoes complicated processes such as Ostwald ripening, aggregation, and/or fusion mechanisms. 21,25) Thus, further optimizations on the choice of ligands and the removal of the polar solvent after the synthesis are required.
Herein, we report a facile LAPR method for size-controlled CsPbBr3 PeQDs exhibiting deep-blue emission with narrow bandwidth by employing didodecyl dimethyl ammonium bromide (DDAB) as an aprotic ligand during the synthesis. So far, the DDAB has been used as a post-treatment agent to prevent aggregation of PeQDs through ligand exchange during solution aging. 13,25,26) However, the aggregation/fusion occurs even during the crystal growth, resulting in less color-tunability and stability of the colloidal PeQDs. To this end, in this contribution, DDAB was added in the poor solvents before the injection of the precursor solution. Despite such a simple modification, blue-emitting quantum-confined CsPbBr3 PeQD (size of ∼4.9 nm) with a narrow bandwidth (∼20.5 nm) has been obtained under ambient conditions (at room temperature in the air). In addition to this, we employed gel permeation chromatography (GPC) 27,28) to remove more than 70% of the polar solvents and confirmed that it significantly improves the stability of the colloidal PeQDs in a liquid phase. Finally, we demonstrated that thin solid films of the PeQDs exhibit similar optical properties to the solution phase, which is a promising result for future LED applications.
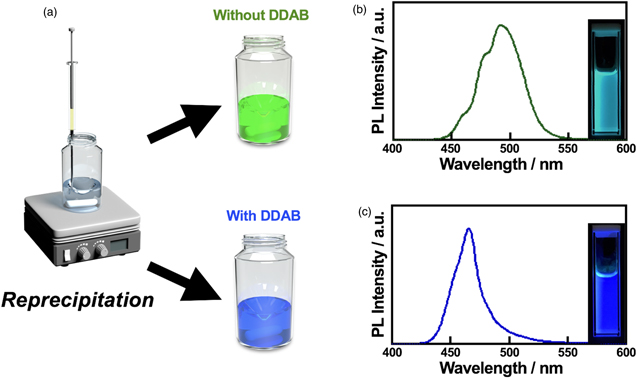
Figure 1(a) schematically illustrates the LARP method for CsPbBr3 PeQDs colloidal synthesis. 19) In brief, a small portion of a precursor solution of CsBr and PbBr2 in a good solvent (dimethylformamide (DMF)) is rapidly injected into a vigorously stirred poor-solvent (e.g. toluene) containing n-octanoic Acid (OA) and n-octylamine (OAm) in the absence or presence of DDAB at room temperature. (The detail of the synthetic procedure and the characterization methods are described in the supporting information, available online at stacks.iop.org/APEX/15/065002/mmedia). The solution turns bluish immediately after the precursor injection, indicating rapid nucleation and crystal growth. Before subjecting the final products to device fabrications, the solutions need to be purified to remove large crystals and the excess amount of reagents for example by centrifugation which typically takes more than 30 min. It has to be noted that the PeQDs undergo complex crystal growth processes during the purification processes because of the presence of the good solvent from the precursor solution, which is DMF in this case. Figures 1(b) and 1(c) show photographs and luminescence spectra of typical PeQDs solutions synthesized in the absence and presence of DDAB, respectively. The result indicates that the presence of DDAB affects the emission properties of the final products, indicating a significant difference in the crystal growth of CsPbBr3 PeQDs.
Fig. 1. (Color online) (a) A schematic illustration of the LARP method. Rapid injection of a precursor solution containing CsBr and PbBr2 in poor solvents containing OA and OAm in the absence and presence of DDAB provides the different color of CsPbBr3 PeQDs solution. Photoluminescence spectra and photographs of the PeQDs without (b) and with DDAB (c), respectively.
Download figure:
Standard image High-resolution imageFor further analysis, the supernatants of the two PeQDs after centrifugations were subjected to TEM, X-ray diffraction (XRD), 1H-NMR measurements. TEM images reveal that both conditions provide spherical PeQDs with different size and dispersity [Figs. 2(a) and 2(b)]. The average size of PeQDs capped with DDAB (DDAB-capped PeQDs) was measured to be 4.9 ± 0.51 nm (well below the Bohr radius of ∼7 nm 10,13,29)) [Fig. 2(c)], while PeQDs without DDAB (non-capped PeQDs) was 7.0 ± 0.80 nm [Fig. 2(d)]. This result indicates that DDAB leads to higher monodispersity. As shown in Fig. S1, non-capped DDAB PeQDs precipitated by centrifugation, and thus the supernatant does not show the PeQD-derived emission and absorption. On the other hand, the supernatant of the DDAB-capped PeQDs shows the emission and absorption corresponding to nanometer-sized PeQDs as shown in Fig. S1. The XRD pattern of the DDAB-capped PeQDs shows Bragg diffraction peaks at 15.01°, 21.43°, 30.48° and 33.02° (Fig. S2), corresponding to (100), (110), (200), and (210) diffraction planes, namely the formation of the cubic CsPbBr3 [ICSD #29073]. The 1H-NMR analysis confirmed resonance peaks of DDAB at 3.2–3.5 ppm (Fig. S3), implying that the PeQDs were passivated by DDAB.
Fig. 2. (Color online) TEM analysis of the CsPbBr3 PeQDs. Typical TEM image of non-capped (a) and DDAB-capped CsPbBr3 PeQDs (b). The corresponding size distributions of non-capped (c) and DDAB-capped PeQDs (d).
Download figure:
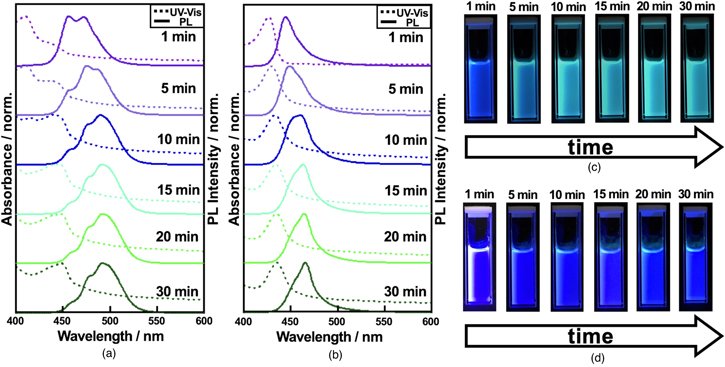
Standard image High-resolution imageThe stability of the non-capped and the DDAB-capped PeQDs in solution represents a significant difference, which is reflected in the time course of UV–vis absorption and PL spectra [Figs. 3(a) and 3(b)]. The corresponding photographs of the solutions under UV irradiation were displayed in Figs. 3(c) and 3(d). The non-capped PeQDs showed blue-emission peaked at 457 and 472 nm at 1 min after the precursor injection, followed by a significant redshift to ∼490 nm with shoulders at ∼475 and ∼460 nm within 30 min [Fig. 2(a)]. The multiple peaks suggest that the suspension contains different sizes of PeQDs that are consistent with the TEM analysis [Fig. 1(c)]. This is typical behavior of PeQDs capped with the mixed ligands of OA and OAm. 23,24) In contrast, PL of the DDAB-capped PeQDs shows a main peak at ∼445 nm and a subpeak at 465 nm at 1 min, while the peak at 465 nm becomes dominant within 30 min [Fig. 2(b)]. The FWHM of each peak is as narrow as ∼20.5 nm. The detailed analysis is summarized in Figs. S4, S5, and Tables SI, SII. The PLQY of the purified DDAB-capped PeQDs was measured to be 86.5%. It is worth mentioning that the final emission of the DDAB-capped PeQDs locates at 2.66 eV (∼465 nm), which is remarkably higher than that of the bulk CsPbBr3 (2.34 eV, i.e. ∼530 nm), 10) indicating the successful control of the quantum confinement effect.
Fig. 3. (Color online) In-situ PL and absorption spectra of (a) CsPbBr3 PeQDs without DDAB and (b) CsPbBr3 PeQDs with DDAB for 30 min. Photographs of (c) colloidal CsPbBr3 PeQD without DDAB and (d) colloidal CsPbBr3 PeQD with DDAB for 30 min.
Download figure:
Standard image High-resolution imageThe concentration of DDAB plays an important role in the passivation of PeQDs. Thus, we have clarified the concentration dependence of DDAB during aging the solution after the LARP. Figures S6–S8 and Tables SIII–SV show a change in PL intensity and peak position, and UV–vis absorption spectrum as a function of time in the solution containing 0, 250, and 500 μl of DDAB toluene solution (0.05 M) for 0.33 and 0.40 mmol of CsBr and PbBr2, respectively. Note that the solution also contains 500 μl of the good solvent, DMF, which causes the proton exchange between the ligands and thus induces destabilization of PeQDs. 30,31) PL intensity of the non-capped PeQDs gradually increases with a significant redshift of the peak position, indicating slow and complicated crystal growth (Fig. S6). This is likely because the protonic ligands cannot stabilize the PeQDs surface sufficiently due to the proton exchange effect, which could induce complicated crystal growth processes including QDs decomposition, Ostwald ripening and aggregation/fusion mechanism. In contrast, in the presence of 250 μl of DDAB toluene solution (the optimal concentration), despite the PL peak redshifts as aforementioned, its intensity stays similar before and after the aging process. Importantly, no obvious redshift of the PL position as well as the change in PL intensity was not observed after 30 min (Fig. S7). This indicates that the PeQDs are well protected by DDAB. A higher amount of DDAB (500 μl of DDAB toluene solution) could suppress the PL redshift over 30 min. However, the PL intensity significantly decreases as a function of the aging time [Fig. S8(a)]. Since the absorbance also decreases without redshifts [Fig. S8(b)], we speculate that the PeQDs slowly decomposed in the solution due to the excess amount of DDAB. 32,33) Thus, the concentration of DDAB is critical for stable mono-dispersed blue-emitting single-halide PeQDs.
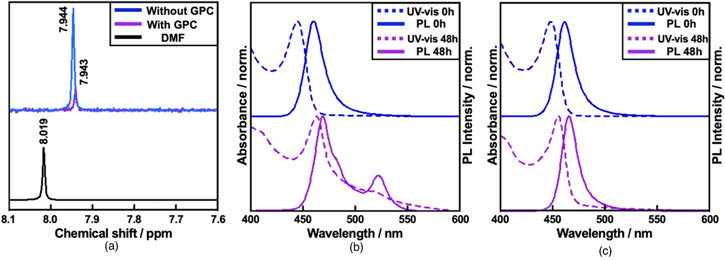
The effect of the polar solvent (DMF) in the reaction mixture on the colloidal stability was evaluated (Fig. 4). The supernatant of the DDAB-capped solution was further purified by means of GPC. 27,28) The 1H-NMR analysis confirmed resonance peaks of DMF at 8.1–7.9 ppm in the colloidal solutions before and after the GPC. However, it revealed that more than 70% of DMF was removed by GPC purification [Fig. 4(a) and Table SVI]. Without removing DMF from the suspension, another PL peak appears around 530 nm within 48 h [Fig. 4(b)], indicating degradation and aggregation/fusion of PeQDs. In contrast, after GPC, their optical properties remain intact for 48 h; the PL peak at 462 nm with FWHM of ∼20 nm and the PLQYs over 80% [Fig. 4(c)]. After a week, without GPC, blue emission disappeared, and green emission only remained [Fig. S9(a)]. On the other hand, with GPC, blue emission was maintained [Fig. S9(b)]. This result indicates that the removal of DMF is a crucial protocol for long-term storage in colloidal states, which is important for device applications.
Fig. 4. (Color online) (a) 1H-NMR in CDCl3 of DMF in DDAB-capped PeQD colloids without and with GPC. PL and absorption spectra of DDAB-capped PeQDs over time (b) without and (c) with GPC.
Download figure:
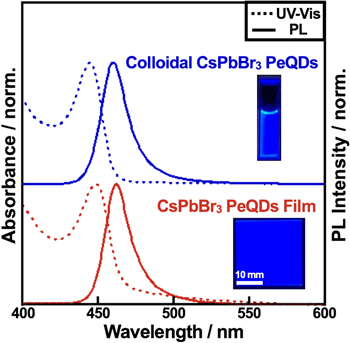
Standard image High-resolution imageFinally, the PeQDs were evaluated in a solid thin film. The thin film was fabricated using a simple spin-coating. After the GPC purification, the colloidal solution was condensed using an evaporator and subjected to the spin-coating process. The UV–vis and PL spectra along with the photographs of the colloidal and the film were displayed in Figs. 5 and S11. It is remarkable that no changes in UV–vis and PL spectra after the film formation. The PLQY, on the other hand, slightly decreased to 56% (Table SIX). However, the stability of the DDAB-capped PeQDs is high enough for film applications. The results presented here indicate that the quantum-confined single-halide PeQDs prepared in this study are suitable for the next-generation photonic source.
Fig. 5. (Color online) PL (solid) and absorption (dashed) spectra of a colloidal solution (top) and a solid film (bottom) of the DDAB-capped CsPbBr3 PeQDs, respectively.
Download figure:
Standard image High-resolution imageIn conclusion, we have demonstrated a simple LARP method in which DDAB could be used to control the nucleation and the crystal growth process to produce blue-emitting CsPbBr3 PeQDs. Adding an appropriate amount of DDAB in the reaction solution of the LAPR synthesis, blue-emitting single-halide PeQDs with a narrow FWHM and high PLQY was successfully obtained. We also presented that removal of the polar solvents from the reactant plays a crucial role in the colloidal stability for long-term storage. Furthermore, the thin film of the DDAB-capped PeQDs retains excellent optical properties, namely a single-peak blue emission with 56% of PLQY, which is a promising performance for the next generation photonic sources. Thus, this work opens a venue for better control of the quantum confinement effect of various PeQDs.
Acknowledgments
This work was performed under the Research Program of "Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials" in "Network Joint Research Center for Materials and Devices."
Supplementary data (4.2 MB, PDF)