Abstract
Uncertainty about future threat has been found to be associated with an overestimation of threat probability and is hypothesized to elicit additional allocation of attention. We used event-related potentials to examine uncertainty-related dynamics in attentional allocation, exploiting brain potentials’ high temporal resolution and sensitivity to attention. Thirty participants performed a picture-viewing task in which cues indicated the subsequent picture valence. A certain-neutral and a certain-aversive cue accurately predicted subsequent picture valence, whereas an uncertain cue did not. Participants overestimated the effective frequency of aversive pictures following the uncertain cue, both during and after the task, signifying expectancy and covariation biases, and they tended to express lower subjective valences for aversive pictures presented after the uncertain cue. Pictures elicited increased P2 and LPP amplitudes when their valence could not be predicted from the cue. For the LPP, this effect was more pronounced in response to neutral pictures. Uncertainty appears to enhance the engagement of early phasic and sustained attention for uncertainly cued targets. Thus, defensive motivation related to uncertainty about future threat elicits specific attentional dynamics implicating prioritization at various processing stages, especially for nonthreatening stimuli that tend to violate expectations.
Similar content being viewed by others
Accurate predictions of the near future are essential in decision making and goal-directed behavior, since they enable us to achieve desirable and avoid undesirable outcomes. Conditions of unpredictable negative events are associated with sustained anxiety and apprehension (Davis, Walker, Miles, & Grillon, 2010; Grillon, 2008; Grupe & Nitschke, 2013) and give rise to the search for strategies to reduce uncertainty—for example, by correctly assessing contingencies (Alloy & Tabachnik, 1984). However, the ability to assess contingencies is impaired, as is indicated by research demonstrating illusory correlations between cues ambiguously signaling potential threat and subsequent aversive events (Grupe & Nitschke, 2011; Sarinopoulos et al., 2010; Wiemer, Mühlberger, & Pauli, 2014). Sarinopoulos and colleagues utilized a passive picture-viewing paradigm in which a cue indicated whether a subsequent picture would be neutral or aversive (certain conditions), or either of the two (uncertain condition). Neutral and aversive pictures occurred equiprobably in the uncertain condition, but participants were not informed of this fact. The authors reported a covariation bias for uncertain cues and aversive pictures—that is, after the experiment, subjects overestimated the proportion of negative pictures in the uncertain condition. Grupe and Nitschke (2011), employing the same paradigm, recorded trial-by-trial ratings of expectancy for negative pictures after each cue, and found an expectancy bias for negative pictures after the uncertain cue.
Furthermore, the biased evaluation of threat probability and the inability to prepare oneself imply that unpredictable emotional events elicit stronger affective responses than do predictable ones. Indeed, this has been demonstrated for various levels of emotional responding, including self-reported mood, valence, or pain intensity (Bar-Anan, Wilson, & Gilbert, 2009; Grupe & Nitschke, 2011; Ploghaus et al., 2001; Yoshida, Seymour, Koltzenburg, & Dolan, 2013) and autonomic reactivity (Grupe & Nitschke, 2011). Similarly, functional magnetic resonance imaging (fMRI) studies have revealed increased uncertainty-related activity in the amygdala and insula (Dunsmoor, Bandettini, & Knight, 2008; Ploghaus et al., 2001; Sarinopoulos et al., 2010; Yoshida et al., 2013), brain regions associated with the processing of emotional salience (Davis & Whalen, 2001; Straube & Miltner, 2011). Moreover, evidence from threat-of-shock paradigms has suggested that uncertainty elicits defensive motivation, which is associated with increased physiological reactivity and hypervigilance. For example, the startle reflex—a somatic marker of defensive motivation (Lang, Bradley, & Cuthbert, 1992)—is enhanced during the anticipation of unpredictable as compared to predictable shocks (Bradford, Shapiro, & Curtin, 2013; Grillon, Baas, Lissek, Smith, & Milstein, 2004; Hefner & Curtin, 2012; Nelson & Shankman, 2011; Shankman, Robison-Andrew, Nelson, Altman, & Campbell, 2011), and fMRI research involving similar contrasts has indicated sustained hyperactivity in attentional networks (Carlsson et al., 2006; Hasler et al., 2007). Consistently, unpredictable threat of shock facilitates early perceptual and attentional processing, even with uncertainty-independent neutral stimuli (Baas, Milstein, Donlevy, & Grillon, 2006; Cornwell et al., 2007; Nelson, Hajcak, & Shankman, 2015; Shackman, Maxwell, McMenamin, Greischar, & Davidson, 2011). Thus, uncertainty-related defensive motivation is strongly tied to enhanced attentive stimulus processing, possibly aiming at threat elimination.
However, it is still unclear how uncertainty affects attentional allocation during target (i.e., “uncertainly cued targets”) processing, especially with regard to its time course. Defensive emotional responding, as indicated by fMRI and autonomic measures (Grupe & Nitschke, 2011; Sarinopoulos et al., 2010; Williams et al., 2015), implies that uncertainly cued targets elicit sustained elaboration (Amrhein, Mühlberger, Pauli, & Wiedemann, 2004; Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Liu, Huang, McGinnis-Deweese, Keil, & Ding, 2012), contrasting with the aforementioned early responses to uncertainty-independent neutral stimuli (Baas et al., 2006; Cornwell et al., 2007; Nelson et al., 2015; Shackman et al., 2011). Therefore, we utilized event-related potentials (ERPs), exploiting their excellent temporal resolution and sensitivity to attentional processes, to investigate the uncertainty-related dynamics in attentional allocation and sustained stimulus elaboration in a cued-picture paradigm (Grupe & Nitschke, 2011; Sarinopoulos et al., 2010).
The anterior P2 is a fronto-central positive deflection around 200 ms after stimulus presentation. As an indicator of early selective attention, it has been shown to index feature detection processes in nonaffective stimuli (Luck & Hillyard, 1994), but also to be enhanced for emotionally relevant pictures (Carretié, Hinojosa, Martín-Loeches, Mercado, & Tapia, 2004; Carretié et al., 2013; Foti & Hajcak, 2008). The late positive potential (LPP) is a centro-parietal slow wave beginning in the P3 time window, around 300 ms after stimulus presentation, that is more positive for motivationally salient stimuli such as targets or emotional pictures (Cuthbert et al., 2000; Schupp et al., 2000). This component has been consistently shown to last throughout and beyond picture presentation (Codispoti, Mazzetti, & Bradley, 2009; Hajcak & Olvet, 2008). Furthermore, apart from the intrinsically arousing quality of emotional pictures, the LPP seems to be modulated by cognitive factors, such as working memory load (MacNamara, Ferri, & Hajcak, 2011), target status (Weinberg, Hilgard, Bartholow, & Hajcak, 2012), directed attention (Hajcak, MacNamara, Foti, Ferri, & Keil, 2013), and emotion regulation (Hajcak & Nieuwenhuis, 2006; Paul, Simon, Kniesche, Kathmann, & Endrass, 2013). Although it has been proposed that the LPP reflects interactions of bottom-up attentional allocation and top-down sustained elaboration (Weinberg, Ferri, & Hajcak, 2013), previous research suggested functional differences between earlier and later portions of the LPP, with stronger top-down influences as stimulus processing progresses (Foti & Hajcak, 2008; Hajcak & Nieuwenhuis, 2006).
With respect to uncertainty processing, increased P2 and LPP amplitudes have been reported for uncertain cues indicating potential threat, relative to certain cues indicating safety, in threat-of-shock designs (Baas, Kenemans, Böcker, & Verbaten, 2002; Bublatzky & Schupp, 2012; Weymar, Bradley, Hamm, & Lang, 2013). Larger P2 amplitudes were also observed for unpredictable than for predictable fearful and neutral faces (Yang, Yuan, & Li, 2012). Furthermore, the later positive components (P3 and LPP) have been shown to be sensitive to stimulus predictability (Picton, 1992), and might therefore be responsive to unpredictable picture valence. Importantly, it has been suggested that the LPP is generated in the brain networks associated with visual and emotional processing (Liu et al., 2012; Sabatinelli, Keil, Frank, & Lang, 2013), including regions that have been shown to be modulated by uncertainty (Sarinopoulos et al., 2010; Williams et al., 2015). Thus, we chose the P2 as an early, more phasic indicator, and the LPP as a later indicator of sustained attentional processing in the present study.
In this study, we investigated uncertainty-related threat estimates and the dynamics of attentional processing through the use of subjective ratings of event frequencies and aversiveness as behavioral indices, as well as ERPs as psychophysiological measures of attentional dynamics. Neutral and aversive pictures were preceded by cues indicating whether the following picture was going to be neutral, aversive, or unpredictably either of the two. For the ERP measures, we hypothesized that picture-locked P2 and LPP amplitudes would be increased for pictures occurring after an uncertain, as compared to a certain, cue. In line with previous research, we expected to find expectancy and covariation biases, as indicated by trial-by-trial expectancy ratings and postexperiment estimates, respectively, exceeding the true frequency of aversive pictures after the uncertain cue (50 %). Furthermore, we expected more negative ratings of subjective valence for aversive pictures under uncertainty.
Method
Sample
The sample size was determined through power analysis using G*Power 3.1.5 (Faul, Erdfelder, Buchner, & Lang, 2009). Our main contrast of interest pertained to the LPP response to pictures in the certain versus the uncertain context, where we intended to detect an effect size of d ~ 1.1 using a two-tailed alpha level = .05 based on previous studies (Baas et al., 2002; Weymar et al., 2013). Calculations indicated that this would require 29 participants to achieve 80 % power. To ensure a fully balanced cue–picture mapping (see the Task and procedure section for details), we aimed to recruit 30 participants. Thirty-three individuals participated in this study, including undergraduate psychology students and individuals from the Berlin community recruited through virtual blackboards. The data of three participants were excluded from the analysis due to excessive sleepiness (n = 1) or disregarding the instructions (n = 2)—that is, instead of giving an expectancy rating (see the Task and procedure section for details), they indicated their preferred subsequent picture valence. The final sample included 30 participants (22 female, eight male) between 18 and 43 years old (M = 25.6, SD = 7.8) that either had obtained or were currently obtaining a university degree (years of education: M = 12.6, SD = 0.75). All participants had normal or corrected-to-normal vision. They gave informed consent according to the Declaration of Helsinki and received either course credit or €10 per hour for participation in this study. The study protocol was approved by the local ethics committee.
Stimulus material
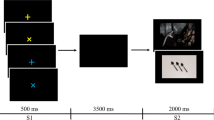
We selected 54 aversive and 54 neutral pictures from the International Affective Pictures System (IAPS; Lang et al., 2008).Footnote 1 According to normative ratings, the aversive pictures were less pleasant (valence M = 2.80, SD = .38) and more arousing (arousal M = 6.05, SD = 0.64) than the neutral pictures (valence, M = 4.98, SD = 0.28; arousal, M = 2.80, SD = 0.55). We used white “O,” “X,” and “?” characters as cues. The pictures occupied 9.7° × 12.9°, and the cues 1.4° × 1.4° of visual angle. All stimuli were presented on a black background.
Task and procedure
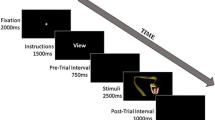
The subjects completed a cued passive picture-viewing task. As can be seen in Fig. 1, on each trial, one of three cues was presented for 2,000 ms. Whereas “O” or “X” indicated certainly neutral or aversive images, respectively, a question mark (“?”) signaled that either a neutral or an aversive picture would unpredictably follow. After each cue, subjects indicated their expectancy of the type of the following picture. On this scale, 0 indicated that they expected a neutral picture to follow with absolute certainty (labeled: definitely neutral), and 100 indicated that they expected an aversive picture with absolute certainty (labeled: definitely negative). These data are referred to as the trial-by-trial expectancy ratings. After an interstimulus interval (ISI; mean duration = 3,000 ms, range = 1,000 to 5,000 ms) featuring a black screen and a white fixation cross in the center, an IAPS picture was presented for 2,000 ms. Another ISI (mean duration = 2,000 ms, range = 1,000 to 3,000 ms) preceded a rating, in which subjects indicated their subjective valence after viewing the picture using a self-assessment manikin (Bradley & Lang, 1994), ranging from –4 (labeled: very bad) to 4 (labeled: very good). Both the expectancy and valence rating prompts were presented until response onset (using a computer mouse), but for a minimum of 3,000 ms. The intertrial intervals ranged from 1,000 to 5,000 ms (mean duration = 3,000 ms).
The task consisted of 108 trials presented in six blocks of 18 trials each, and took approximately 30 min to complete. Neutral and aversive pictures appeared equiprobably across the entire task (each individual picture was presented only once). Whereas the aversive and neutral cues were always followed by the respective picture type (certain-aversive and certain-neutral conditions, N = 36 trials each), the uncertain cue was followed on half of the trials by an aversive picture (uncertain-aversive condition, N = 18 trials) and on the remaining trials by a neutral picture (uncertain-neutral condition, N = 18 trials). Subjects were explicitly instructed about the predictive values of the certain cues, but they were only told that the uncertain cue would be followed by either a neutral or an aversive picture, without telling the exact contingencies. To ensure equally valenced and arousing images in the certain and uncertain trials, we divided both the neutral and the aversive picture sets into three subsets of 18 that did not vary with respect to normative valence and arousal ratings (all Fs ≤ 1.05, all ps > .35). For each subject, we assigned two neutral subsets to the certain-neutral cue, two aversive subsets to the certain-aversive cue, and one of each to the uncertain cue. This procedure was balanced across subjects. The order of picture presentation was randomized, but never were more than two cues of the same category presented in a row.
The task was performed in a dimly lit, air-conditioned, and electrically shielded room, where participants were seated in front of a 19-in. screen at a viewing distance of 85 cm. After the task, subjects estimated the percentage of uncertain cues that were followed by an aversive picture. This was again accomplished on a scale ranging from 0 to 100. These data are referred to as the postexperiment estimates (cf. Grupe & Nitschke, 2011).
Electroencephalogram (EEG) recording and data reduction
During the task, an EEG was continuously recorded with Ag/AgCl electrodes from 60 sites from a concentric and equidistant EasyCap montage (Easycap GmbH, Breitbrunn, Germany), as well as from four external positions: approximately 2 cm below each eye, the nasion, and the neck. The impedances of all electrodes were kept below 5 kΩ. All electrodes were referenced to Cz, and an electrode attached to the right cheek served as the ground electrode. The EEG was amplified with two 32-channel BrainAmp amplifiers (Brain Products GmbH, Munich, Germany) at a digitization rate of 250 Hz using a band-pass filter of 0.01–500 Hz.
We conducted offline analyses using the BrainVision Analyzer 2.0 software package (Brain Products GmbH, Munich, Germany). The raw data were re-referenced to a computed average of T9 and T10 recordings, after ocular artifacts were removed utilizing the multiple-source eye correction method (i.e., the Surrogate Method; Berg & Scherg, 1994) in BESA 6.0 (Brain Electrical Source Analysis; MEGIS Software GmbH, Gräfeling, Germany). Since the LPP is a broadly distributed component, we pooled six centro-parietal electrodes—CP1, CP2, CPz, P1, P2, and Pz—where valence effects were most strongly pronounced. For the P2, we pooled six fronto-central electrodes—FC1, FC2, FCz, F1, F2, and Fz—on the basis of visual inspection. After digital band-pass filtering with Butterworth zero-phase filters between 0.1 Hz (time constant 1.59 s) and 30 Hz (half-power frequencies, slopes: 24 dB/octave), we created trial-based epochs between –200 and 2,000 ms relative to the picture onsets. Single-trial epochs with artifacts including voltage steps per sampling point exceeding 50 μV, absolute amplitude differences exceeding 300 μV within a 200-ms window, or low activity with voltage changes of less than 0.5 μV within a 100-ms window were automatically excluded from further analyses. We applied a baseline correction using the 200-ms prestimulus window and calculated individual picture-locked averages for each cue–picture combination (certain neutral, certain aversive, uncertain neutral, and uncertain aversive). The mean numbers of trials included in the averages (with standard deviation; and percentage of excluded trials) were 35.0 (2.1; 2.7 %) for certain-neutral, 35.2 (1.2; 2.3 %) for certain-aversive, 17.8 (0.6; 1.1 %) for uncertain-neutral, and 17.7 (0.7; 1.7 %) for uncertain-aversive trials. To account for the potentially differential influences of bottom-up and top-down processes on attentional allocation, we extracted the mean amplitudes of the picture-locked LPP in the 400- to 1,000-ms (early LPP) and 1,000- to 2,000-ms (late LPP) time windows. For the P2, we first determined the most positive peak in the 140- to 200-ms time window, and then extracted means around this peak (±30 ms).
Statistical analyses
To assess expectancy and covariation biases, the mean trial-by-trial expectancy ratings and the postexperiment estimates, respectively, were subjected to one-sample t tests to examine whether they exceeded 50 (true frequency of the aversive pictures). The critical p value for these one-sided tests was set to p = .025. To estimate uncertainty-induced modulations of self-reported emotional responding, a two-way repeated measures analysis of variance (ANOVA) was conducted on the ratings of subjective valence, with Valence (neutral, aversive) and Certainty (certain, uncertain) as within-subjects factors. Two-way repeated measures ANOVAs on the picture-locked P2 amplitudes with Valence (neutral, aversive) and Certainty (certain, uncertain) as within-subjects factors served to assess uncertainty-related modulation in early attention. For effects on sustained processing, we analyzed the LPP amplitudes with a three-way repeated measures ANOVA using Valence (neutral, aversive), Certainty (certain, uncertain), and Time Window (early, late) as within-subjects factors. Significant interactions were followed up by post-hoc t tests using Bonferroni correction. Effect sizes are provided as partial eta-squared (η p 2) for F tests and as Cohen’s d for t tests.
Results
Behavioral data
Expectancy ratings after the certain-neutral (M = 0.45, SD = 1.52) and certain-aversive (M = 99.46, SD = 1.90) cues indicated that their predictive function was reliably identified. One-sample t tests comparing the trial-by-trial expectancy ratings after the uncertain cue and the postexperiment estimates with the true frequency of 50 % revealed that participants overexpected aversive pictures, t(29) = 2.75, p = .019, d = 0.45 (M = 52.99, SD = 6.61), and overestimated the overall frequency of aversive pictures, t(29) = 2.66, p = .013, d = 0.48 (M = 56.70, SD = 13.88).
A 2 (Valence) × 2 (Certainty) repeated measures ANOVA on the subjective valence ratings yielded a significant main effect of valence, F(1, 29) = 185.11, p < .001, η p 2 = .87, indicating that subjective valence was more negative after aversive pictures. However, we found no significant certainty effect, F(1, 29) = 2.727, p = .109, η p 2 = .09, and no interaction of valence and certainty, F(1, 29) = 1.45, p = .239, η p 2 = .05. A post-hoc test comparing valence ratings for the certain and uncertain aversive pictures reached trend level, t(29) = 1.74, p = .093, d = 0.17, indicating a tendency for more negative valence ratings after the uncertain cue. Ratings for neutral pictures did not differ between the certainty conditions, t(29) = 0.09, p = .93, d < 0.01).
Picture-locked P2 and LPP
Figure 2 Footnote 2 displays picture-locked P2 and LPP waveforms and topographies. The 2 (Valence) × 2 (Certainty) repeated measures ANOVA on the picture-locked P2 amplitudes yielded a significant main effect of certainty, F(1, 29) = 7.72, p = .009, η p 2 = .21, indicating that P2 amplitudes were more positive for pictures after uncertain than after certain cues. P2 amplitudes did not differ with respect to valence, F(1, 29) = 0.05, p = .827, η p 2 = .002, nor was there an interaction, F(1, 29) = 0.03, p = .866, η p 2 = .001. The 2 (Valence) × 2 (Certainty) × 2 (Time Window) repeated measures ANOVA on the picture-locked LPPs yielded a significant main effect of valence, F(1, 29) = 27.85, p < .001, η p 2 = .49, indicating that LPP amplitudes were more positive for aversive than for neutral pictures. Importantly, we also observed a significant main effect of certainty, F(1, 29) = 22.42, p < .001, η p 2 = .44, indicating that LPP amplitudes were more positive for pictures after uncertain cues. The certainty effect was modulated by picture valence, as certainty significantly interacted with valence, F(1, 29) = 4.61, p = .040, η p 2 = .14, and it varied over time, as was revealed by a significant interaction of certainty with time, F(1, 29) = 8.23, p = .008, η p 2 = .22. Post-hoc tests showed that the certainty effect was slightly diminished, but still significant, for aversive pictures (neutral: p < .001, d = 0.86; aversive: p = .010, d = 0.39) and in the late time window (early: p < .001, d = 0.66; late: p = .002, d = 0.54). No further main effects or interactions reached significance, ps > .26, η p 2s < .05.
Display of the certainty effect, indicated by P2 and late positive potential (LPP) amplitudes. (A) Picture-locked event-related potential (ERP) waveforms averaged at FC1, FC2, FCz, F1, F2, and Fz for certain-neutral, certain-aversive, uncertain-neutral, and uncertain-aversive trials. (B) Topographic maps displaying voltage differences in the 150- to 210-ms time range between uncertain-aversive and certain-aversive trials (left), and between uncertain-neutral and certain-neutral trials (right). (C) Picture-locked ERP waveforms averaged at CP1, CP2, CPz, P1, P2, and Pz for certain-neutral, certain-aversive, uncertain-neutral, and uncertain-aversive trials. The data were low-pass-filtered at 8 Hz for viewing purposes only. (D) Topographic maps displaying voltage differences between uncertain-aversive and certain-aversive trials (left) and between uncertain-neutral and certain-neutral trials (right) in the 400- to 1,000-ms (top) and the 1,000- to 2,000-ms (bottom) time ranges.
Discussion
In this study, we examined negativity bias and dynamics in attentional allocation under uncertainty about potential threat. In line with previous studies, we found expectancy and covariation biases; that is, participants overestimated the frequency of aversive pictures under uncertainty, as well as showed a tendency for more negative subjective valences in response to aversive pictures following uncertain than following certain cues. At the electrophysiological level, we observed that picture-locked P2 and LPP amplitudes showed a significant increase for stimuli presented after uncertain relative to certain cues. For the LPP, this effect persisted throughout stimulus presentation. Whereas the P2 was selectively responsive to uncertainty, additive effects of uncertainty and valence were observed for the LPP, with larger amplitudes occurring for pictures presented after uncertain cues and for aversive pictures. Furthermore, the uncertainty effect in the LPP was more pronounced in response to neutral pictures.
As hypothesized, we observed larger P2 and LPP amplitudes for pictures after uncertain than after certain cues. The effect on P2 amplitudes extends previous research (Grupe & Nitschke, 2011; Sarinopoulos et al., 2010) by suggesting that uncertainly cued targets already enhance early selective attention. A similar P2 amplitude increase was observed for faces without versus with cued valence (Yang et al., 2012). Furthermore, increased attention continued during the LPP interval. This elucidates important differences between the attentional dynamics to uncertainly cued targets, which we examined here, and stimuli encountered under conditions of unpredictable threat but otherwise noninformative about and unrelated to uncertainty. The latter stimuli tend to elicit an attentional response characterized by increased early and decreased later resource allocation (Baas et al., 2006; Cornwell et al., 2007; Nelson et al., 2015; Shackman et al., 2011), which has been interpreted as rapid stimulus discrimination—facilitating the resolution of uncertainty—but subsequent disengagement after categorizing a stimulus as irrelevant to impending threat. Contrarily, as we presently observed, the uncertainty-related LPP does not normalize during the presentation of uncertainly cued targets, reflecting a sustained attentional response that is unlikely to facilitate the resolution of uncertainty through valence discrimination. Hence, prolonged picture engagement suggests that targets remain highly relevant (Ferrari, Codispoti, Cardinale, & Bradley, 2008; Schupp et al., 2007; Weinberg et al., 2012) and that additional attentional resources—facilitated by uncertainty-related defensive engagement—are fully deployed to serve not only early examination, but also higher-level cognitive processing (Dolcos & Cabeza, 2002; Koenig & Mecklinger, 2008). The increased attentional resources under uncertainty appear to be flexibly deployed—that is, persistently allocated or rapidly redirected, according to circumstantial demands.
Another key finding of the present study is that picture type modulated uncertainty-related increases in attention as a function of processing stage. Interestingly, P2 amplitudes were affected by uncertainty only, which contrasts with previously reported modulations by highly arousing stimuli (Carretié et al., 2004; Carretié et al., 2013; Foti & Hajcak, 2008). Apparently, the uncertainty-induced increase in early attentional capture cannot be further modulated by selective processing of neutral and aversive stimuli. Moreover, whereas picture type did affect later attentional processing, as could be expected (Cuthbert et al., 2000; Schupp et al., 2000; Weinberg & Hajcak, 2010), uncertainty continuously evoked additional resource allocation. However, while this increase in attention was not specific to picture type, suggesting a generally enhanced attentional response to uncertainly cued targets, the LPP was more pronounced for neutral pictures. This might reflect a ceiling effect, with certain aversive pictures already capturing a considerable amount of attentional resources and leaving less room for uncertainty-related modulations than was available with certain neutral pictures, which were associated with relatively low attentional capture. One way to test this hypothesis in the future would be to contrast pictures of low (e.g., animal threat) and high (e.g., mutilations) arousal—the latter of which elicit higher LPP amplitudes (Weinberg & Hajcak, 2010)—and compare the increases in LPP amplitude under uncertainty. Alternatively, in the context of behaviorally observed overestimation of threat, this might indicate that targets estimated as being less likely to occur are of special interest and more intensely recruit uncertainty-related processing. As such, ongoing elaboration of neutral targets that deviate from expectation may reflect continued monitoring for threat. This interpretation implies another significant aspect of uncertainty-related attentional dynamics: Attentional allocation driven by uncertainty-induced defensive motivation does not seem to simply constitute an amplified effect of the arousing, attention-grabbing properties inherent to emotional stimuli, but rather appears to operate independently.
However, on this note, it should be pointed out that the presently used stimuli not only differed in valence, but also in arousal. High arousal is common to both negative and positive pictures (Bradley, Codispoti, Cuthbert, & Lang, 2001; Lang, 1995) and is well-known to drive attention, as indicated by the LPP (Cuthbert et al., 2000; Schupp et al., 2000; Schupp et al., 2007; Weinberg & Hajcak, 2010), thus reflecting a potential confound and limiting interpretation of the present data. Indeed, although a magnetoencephalography (MEG) study contrasting negative and positive pictures reported increased neural responses only to uncertain aversive pictures (Onoda et al., 2006)—implying the involvement of a threat-specific component that we were unable to capture—uncertainty may also affect the emotional processing of pleasant stimuli (Bar-Anan et al., 2009). Therefore, additionally including positive material in the future would contribute to disentangling valence- and arousal-related effects on attention under uncertainty.
Our results are inconsistent with a study that failed to reveal uncertainty-related modulations of the LPP in individuals reporting low intolerance of uncertainty (IU), but did show decreased LPPs specifically for uncertain aversive stimuli in individuals reporting high IU (Gole, Schäfer, & Schienle, 2012). However, although a cueing procedure similar to ours was implemented in that study, the participants were explicitly informed that the neutral and aversive pictures occurred equiprobably after uncertain cues. Since IU is associated with increased sensitivity and negative emotion toward uncertainty (Gentes & Ruscio, 2011), this information might have failed to induce sufficient subjective uncertainty, and thus reduced uncertainty-related processes in low-IU individuals. In fact, the lack of any knowledge about the contingencies related to the uncertain cue in the present study may have been an important factor driving attentional allocation. However, the selective avoidance of uncertain threat in high-IU individuals in the Gole et al. study highlights the impact of interindividual differences on uncertainty processing and warrants investigations of patient groups characterized by high IU (Mahoney & McEvoy, 2012; McEvoy & Mahoney, 2012).
We also found a tendency for more negative valence ratings after aversive pictures following uncertain rather than certain cues, consistent with previous reports (Grupe & Nitschke, 2011; Yoshida et al., 2013). Although a larger sample might have shown this more robustly, another possible explanation for this relatively weak effect is that the stimulus materials used in our study may not have been aversive enough to elicit a strong uncertainty-related bias. For example, Wiemer and colleagues (2014) demonstrated that expectancy and covariation biases can be affected by an outcome’s aversiveness. Whereas we presented IAPS pictures of moderate aversiveness, Grupe and Nitschke (2011) included pictures of the most aversive kind, and Yoshida and colleagues (2013) used noxious thermal stimuli. Future research should address this issue by contrasting stimuli of moderate and high aversiveness.
Uncertainty-related expectancy and covariation biases are consistent with previous work (Grupe & Nitschke, 2011; Sarinopoulos et al., 2010; Wiemer et al., 2014). Evidently, unpredictable threat elicits persistent tendencies to perceive illusory correlations between otherwise emotionally neutral cues of low informative value and aversive consequences. Importantly, both biases have been shown to be associated with state anxiety (Wiemer et al., 2014). Since uncertainty-related defensive motivation has been tied to sustained states of anxiety (Davis et al., 2010; Grillon, 2008), the presently observed biases suggest that our participants may, indeed, have engaged a defensive state under uncertainty.
A limitation of the present work pertains to the cues. Due to the use of a question mark as an easily accessible marker for uncertainty, the cueing stimuli were not balanced across conditions. Thus, whereas uncertainty-related attentional and anticipatory modulations have been shown with respect to cue processing (Bublatzky & Schupp, 2012; Herwig, Kaffenberger, Baumgartner, & Jancke, 2007; Sarinopoulos et al., 2010; Weymar et al., 2013; Williams et al., 2015), we could not validly separate effects resulting from the predictive functions and perceptual features of the currently used cues. In fact, a subsequent control study showed similar P2 and LPP effects when the same stimuli were used for a matching task (see the supplementary materials for details). Lastly, the uncertain conditions included relatively few trials (N = 18). Although the LPP appears to be robustly measurable with even fewer epochs (Moran, Jendrusina, & Moser, 2013), future studies should increase the number of trials in order to ensure sufficient signal-to-noise ratios.
In sum, we add to the literature by elucidating uncertainty-related modulations in attentional dynamics. Threat uncertainty context was associated with negatively biased threat estimates and resulted in increased early attentional capture and late top-down allocation of attention to pictures. This later effect was stronger for neutral pictures, and thus went contrary to the effects of picture valence, showing that separable processing mechanisms were at work.
Notes
The following IAPS pictures were used in this study: aversive: 2691, 2811, 3181, 3350, 3500, 3530, 3550_1, 6212, 6230, 6231, 6243, 6250, 6260, 6263, 6312, 6313, 6315, 6350, 6360, 6370, 6510, 6530, 6540, 6550, 6560, 6563, 6570, 6831, 6838, 8485, 9050, 9120, 9250, 9252, 9265, 9413, 9414, 9420, 9421, 9425, 9433, 9500, 9600, 9620, 9623, 9630, 9635_1, 9902, 9908, 9910, 9911, 9920, and 9921; neutral: 2880, 5390, 5395, 5500, 5510, 5520, 5530, 5533, 5534, 5535, 5740, 6150, 7000, 7003, 7004, 7006, 7009, 7010, 7012, 7017, 7025, 7026, 7031, 7043, 7035, 7040, 7041, 7050, 7053, 7059, 7080, 7090, 7100, 7130, 7150, 7161, 7170, 7175, 7185, 7186, 7190, 7205, 7207, 7211, 7217, 7224, 7233, 7235, 7490, 7491, 7500, 7595, 7705, and 7950.
As can be seen in Fig. 2A, uncertain trials already evidence a more positive deflection before the P2 time window, especially in the 0- to 80-ms interval. However, attempts to eliminate this striking deviation by applying different high-pass filters remained unsuccessful. In order to nevertheless establish that the P2 effects reported here are indeed specific to the P2 interval, we additionally compared the certain and uncertain trials in successive 30-ms bins starting after stimulus onset. These additional processing steps and analyses are detailed in the supplementary materials.
References
Alloy, L. B., & Tabachnik, N. (1984). Assessment of covariation by humans and animals: The joint influence of prior expectations and current situational information. Psychological Review, 91, 112–149. doi:10.1037/0033-295x.91.1.112
Amrhein, C., Mühlberger, A., Pauli, P., & Wiedemann, G. (2004). Modulation of event-related brain potentials during affective picture processing: A complement to startle reflex and skin conductance response? International Journal of Psychophysiology, 54, 231–240. doi:10.1016/j.ijpsycho.2004.05.009
Baas, J. M., Kenemans, J. L., Böcker, K. B., & Verbaten, M. N. (2002). Threat-induced cortical processing and startle potentiation. NeuroReport, 13, 133–137.
Baas, J. M., Milstein, J., Donlevy, M., & Grillon, C. (2006). Brainstem correlates of defensive states in humans. Biological Psychiatry, 59, 588–593. doi:10.1016/j.biopsych.2005.09.009
Bar-Anan, Y., Wilson, T. D., & Gilbert, D. T. (2009). The feeling of uncertainty intensifies affective reactions. Emotion, 9, 123–127. doi:10.1037/a0014607
Berg, P., & Scherg, M. (1994). A multiple source approach to the correction of eye artifacts. Electroencephalography and Clinical Neurophysiology, 90, 229–241. doi:10.1016/0013-4694(94)90094-9
Bradford, D. E., Shapiro, B. L., & Curtin, J. J. (2013). How bad could it be? Alcohol dampens stress responses to threat of uncertain intensity. Psychological Science, 24, 2541–2549. doi:10.1177/0956797613499923
Bradley, M. M., Codispoti, M., Cuthbert, B. N., & Lang, P. J. (2001). Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion, 1, 276–298. doi:10.1037/1528-3542.1.3.276
Bradley, M. M., & Lang, P. J. (1994). Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavioral Therapy and Experimental Psychiatry, 25, 49–59. doi:10.1016/0005-7916(94)90063-9
Bublatzky, F., & Schupp, H. T. (2012). Pictures cueing threat: Brain dynamics in viewing explicitly instructed danger cues. Social Cognitive and Affective Neuroscience, 7, 611–622. doi:10.1093/scan/nsr032
Carlsson, K., Andersson, J., Petrovic, P., Petersson, K. M., Öhman, A., & Ingvar, M. (2006). Predictability modulates the affective and sensory-discriminative neural processing of pain. NeuroImage, 32, 1804–1814. doi:10.1016/j.neuroimage.2006.05.027
Carretié, L., Hinojosa, J. A., Martín-Loeches, M., Mercado, F., & Tapia, M. (2004). Automatic attention to emotional stimuli: Neural correlates. Human Brain Mapping, 22, 290–299. doi:10.1002/hbm.20037
Carretié, L., Kessel, D., Carboni, A., López-Martín, S., Albert, J., Tapia, M., & Hinojosa, J. A. (2013). Exogenous attention to facial vs. non-facial emotional visual stimuli. Social Cognitive and Affective Neuroscience, 8, 764–773. doi:10.1093/scan/nss068
Codispoti, M., Mazzetti, M., & Bradley, M. M. (2009). Unmasking emotion: Exposure duration and emotional engagement. Psychophysiology, 46, 731–738. doi:10.1111/j.1469-8986.2009.00804.x
Cornwell, B. R., Baas, J. M. P., Johnson, L., Holroyd, T., Carver, F. W., Lissek, S., & Grillon, C. (2007). Neural responses to auditory stimulus deviance under threat of electric shock revealed by spatially-filtered magnetoencephalography. NeuroImage, 37, 282–289. doi:10.1016/j.neuroimage.2007.04.055
Cuthbert, B. N., Schupp, H. T., Bradley, M. M., Birbaumer, N., & Lang, P. J. (2000). Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology, 52, 95–111. doi:10.1016/S0301-0511(99)00044-7
Davis, M., Walker, D. L., Miles, L., & Grillon, C. (2010). Phasic vs. sustained fear in rats and humans: Role of the extended amygdala in fear vs. anxiety. Neuropsychopharmacology, 35, 105–135. doi:10.1038/NPP.2009.109
Davis, M., & Whalen, P. J. (2001). The amygdala: Vigilance and emotion. Molecular Psychiatry, 6, 13–34.
Dolcos, F., & Cabeza, R. (2002). Event-related potentials of emotional memory: Encoding pleasant, unpleasant, and neutral pictures. Cognitive, Affective, & Behavioral Neuroscience, 2, 252–263. doi:10.3758/CABN.2.3.252
Dunsmoor, J. E., Bandettini, P. A., & Knight, D. C. (2008). Neural correlates of unconditioned response diminution during Pavlovian conditioning. NeuroImage, 40, 811–817. doi:10.1016/j.neuroimage.2007.11.042
Faul, F., Erdfelder, E., Buchner, A., & Lang, A. G. (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41, 1149–1160. doi:10.3758/BRM.41.4.1149
Ferrari, V., Codispoti, M., Cardinale, R., & Bradley, M. M. (2008). Directed and motivated attention during processing of natural scenes. Journal of Cognitive Neuroscience, 20, 1753–1761. doi:10.1162/jocn.2008.20121
Foti, D., & Hajcak, G. (2008). Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience, 20, 977–988. doi:10.1162/jocn.2008.20066
Gentes, E. L., & Ruscio, A. M. (2011). A meta-analysis of the relation of intolerance of uncertainty to symptoms of generalized anxiety disorder, major depressive disorder, and obsessive-compulsive disorder. Clinical Psychology Review, 31, 923–933. doi:10.1016/j.cpr.2011.05.001
Gole, M., Schäfer, A., & Schienle, A. (2012). Event-related potentials during exposure to aversion and its anticipation: The moderating effect of intolerance of uncertainty. Neuroscience Letters, 507, 112–117. doi:10.1016/j.neulet.2011.11.054
Grillon, C. (2008). Models and mechanisms of anxiety: Evidence from startle studies. Psychopharmacology, 199, 421–437. doi:10.1007/s00213-007-1019-1
Grillon, C., Baas, J. P., Lissek, S., Smith, K., & Milstein, J. (2004). Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience, 118, 916–924. doi:10.1037/0735-7044.118.5.916
Grupe, D. W., & Nitschke, J. B. (2011). Uncertainty is associated with biased expectancies and heightened responses to aversion. Emotion, 11, 413–424. doi:10.1037/a0022583
Grupe, D. W., & Nitschke, J. B. (2013). Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nature Reviews Neuroscience, 14, 488–501. doi:10.1038/nrn3524
Hajcak, G., MacNamara, A., Foti, D., Ferri, J., & Keil, A. (2013). The dynamic allocation of attention to emotion: Simultaneous and independent evidence from the late positive potential and steady state visual evoked potentials. Biological Psychology, 92, 447–455. doi:10.1016/j.biopsycho.2011.11.012
Hajcak, G., & Nieuwenhuis, S. (2006). Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective, & Behavioral Neuroscience, 6, 291–297. doi:10.3758/CABN.6.4.291
Hajcak, G., & Olvet, D. M. (2008). The persistence of attention to emotion: Brain potentials during and after picture presentation. Emotion, 8, 250–255. doi:10.1037/1528-3542.8.2.250
Hasler, G., Fromm, S., Alvarez, R. P., Luckenbaugh, D. A., Drevets, W. C., & Grillon, C. (2007). Cerebral blood flow in immediate and sustained anxiety. Journal of Neuroscience, 27, 6313–6319. doi:10.1523/Jneurosci.5369-06.2007
Hefner, K. R., & Curtin, J. J. (2012). Alcohol stress response dampening: Selective reduction of anxiety in the face of uncertain threat. Journal of Psychopharmacology, 26, 232–244. doi:10.1177/0269881111416691
Herwig, U., Kaffenberger, T., Baumgartner, T., & Jancke, L. (2007). Neural correlates of a “pessimistic” attitude when anticipating events of unknown emotional valence. NeuroImage, 34, 848–858. doi:10.1016/j.neuroimage.2006.09.035
Koenig, S., & Mecklinger, A. (2008). Electrophysiological correlates of encoding and retrieving emotional events. Emotion, 8, 162–173. doi:10.1037/1528-3542.8.2.162
Lang, P. J. (1995). The emotion probe: Studies of motivation and attention. American Psychologist, 50, 372–385. doi:10.1037/0003-066x.50.5.372
Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1992). A motivational analysis of emotion—reflex cortex connections. Psychological Science, 3, 44–49. doi:10.1111/j.1467-9280.1992.tb00255.x
Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (2008). International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual (Technical Report No. A-8). Gainesville, FL: University of Florida, Center for Research in Psychophysiology.
Liu, Y., Huang, H., McGinnis-Deweese, M., Keil, A., & Ding, M. (2012). Neural substrate of the late positive potential in emotional processing. Journal of Neuroscience, 32, 14563–14572. doi:10.1523/JNEUROSCI.3109-12.2012
Luck, S. J., & Hillyard, S. A. (1994). Electrophysiological correlates of feature analysis during visual-search. Psychophysiology, 31, 291–308. doi:10.1111/j.1469-8986.1994.tb02218.x
MacNamara, A., Ferri, J., & Hajcak, G. (2011). Working memory load reduces the late positive potential and this effect is attenuated with increasing anxiety. Cognitive, Affective, & Behavioral Neuroscience, 11, 321–331. doi:10.3758/s13415-011-0036-z
Mahoney, A. E., & McEvoy, P. M. (2012). A transdiagnostic examination of intolerance of uncertainty across anxiety and depressive disorders. Cognitive Behavior Therapy, 41, 212–222. doi:10.1080/16506073.2011.622130
McEvoy, P. M., & Mahoney, A. E. (2012). To be sure, to be sure: Intolerance of uncertainty mediates symptoms of various anxiety disorders and depression. Behavior Therapy, 43, 533–545. doi:10.1016/j.beth.2011.02.007
Moran, T. P., Jendrusina, A. A., & Moser, J. S. (2013). The psychometric properties of the late positive potential during emotion processing and regulation. Brain Research, 1516, 66–75. doi:10.1016/j.brainres.2013.04.018
Nelson, B. D., Hajcak, G., & Shankman, S. A. (2015). Event-related potentials to acoustic startle probes during the anticipation of predictable and unpredictable threat. Psychophysiology, 52, 887–894. doi:10.1111/psyp.12418
Nelson, B. D., & Shankman, S. A. (2011). Does intolerance of uncertainty predict anticipatory startle responses to uncertain threat? International Journal of Psychophysiology, 81, 107–115. doi:10.1016/j.ijpsycho.2011.05.003
Onoda, K., Okamoto, Y., Shishida, K., Hashizume, A., Ueda, K., Kinoshita, A., & Yamawaki, S. (2006). Anticipation of affective image modulates visual evoked magnetic fields (VEF). Experimental Brain Research, 175, 536–543. doi:10.1007/s00221-006-0569-5
Paul, S., Simon, D., Kniesche, R., Kathmann, N., & Endrass, T. (2013). Timing effects of antecedent- and response-focused emotion regulation strategies. Biological Psychology, 94, 136–142. doi:10.1016/j.biopsycho.2013.05.019
Picton, T. W. (1992). The P300 wave of the human event-related potential. Journal of Clinical Neurophysiology, 9, 456–479.
Ploghaus, A., Narain, C., Beckmann, C. F., Clare, S., Bantick, S., Wise, R., & Tracey, I. (2001). Exacerbation of pain by anxiety is associated with activity in a hippocampal network. Journal of Neuroscience, 21, 9896–9903.
Sabatinelli, D., Keil, A., Frank, D. W., & Lang, P. J. (2013). Emotional perception: Correspondence of early and late event-related potentials with cortical and subcortical functional MRI. Biological Psychology, 92, 513–519. doi:10.1016/j.biopsycho.2012.04.005
Sarinopoulos, I., Grupe, D. W., Mackiewicz, K. L., Herrington, J. D., Lor, M., Steege, E. E., & Nitschke, J. B. (2010). Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cerebral Cortex, 20, 929–940. doi:10.1093/cercor/bhp155
Schupp, H. T., Cuthbert, B. N., Bradley, M. M., Cacioppo, J. T., Ito, T., & Lang, P. J. (2000). Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology, 37, 257–261. doi:10.1017/S0048577200001530
Schupp, H. T., Stockburger, J., Codispoti, M., Junghofer, M., Weike, A. I., & Hamm, A. O. (2007). Selective visual attention to emotion. Journal of Neuroscience, 27, 1082–1089. doi:10.1523/Jneurosci.3223-06.2007
Shackman, A. J., Maxwell, J. S., McMenamin, B. W., Greischar, L. L., & Davidson, R. J. (2011). Stress potentiates early and attenuates late stages of visual processing. Journal of Neuroscience, 31, 1156–1161. doi:10.1523/JNEUROSCI.3384-10.2011
Shankman, S. A., Robison-Andrew, E. J., Nelson, B. D., Altman, S. E., & Campbell, M. L. (2011). Effects of predictability of shock timing and intensity on aversive responses. International Journal of Psychophysiology, 80, 112–118. doi:10.1016/j.ijpsycho.2011.02.008
Straube, T., & Miltner, W. H. (2011). Attention to aversive emotion and specific activation of the right insula and right somatosensory cortex. NeuroImage, 54, 2534–2538. doi:10.1016/j.neuroimage.2010.10.010
Weinberg, A., Ferri, J., & Hajcak, G. (2013). Interactions between attention and emotion. In M. D. Robinson, E. R. Watkins, & E. Harmon-Jones (Eds.), Handbook of cognition and emotion (pp. 35–54). New York, NY: Guilford Press.
Weinberg, A., & Hajcak, G. (2010). Beyond good and evil: The time-course of neural activity elicited by specific picture content. Emotion, 10, 767–782. doi:10.1037/A0020242
Weinberg, A., Hilgard, J., Bartholow, B. D., & Hajcak, G. (2012). Emotional targets: Evaluative categorization as a function of context and content. International Journal of Psychophysiology, 84, 149–154. doi:10.1016/j.ijpsycho.2012.01.023
Weymar, M., Bradley, M. M., Hamm, A. O., & Lang, P. J. (2013). When fear forms memories: Threat of shock and brain potentials during encoding and recognition. Cortex, 49, 819–826. doi:10.1016/j.cortex.2012.02.012
Wiemer, J., Mühlberger, A., & Pauli, P. (2014). Illusory correlations between neutral and aversive stimuli can be induced by outcome aversiveness. Cognition and Emotion, 28, 193–207. doi:10.1080/02699931.2013.809699
Williams, L. E., Oler, J. A., Fox, A. S., McFarlin, D. R., Rogers, G. M., Jesson, M. A. L., & Kalin, N. H. (2015). Fear of the unknown: Uncertain anticipation reveals amygdala alterations in childhood anxiety disorders. Neuropsychopharmacology, 40, 1428–1435. doi:10.1038/npp.2014.328
Yang, J., Yuan, J., & Li, H. (2012). Expectation decreases brain susceptibility to fearful stimuli: ERP evidence from a modified emotion evaluation task. Neuroscience Letters, 514, 198–203. doi:10.1016/j.neulet.2012.02.094
Yoshida, W., Seymour, B., Koltzenburg, M., & Dolan, R. J. (2013). Uncertainty increases pain: Evidence for a novel mechanism of pain modulation involving the periaqueductal gray. Journal of Neuroscience, 33, 5638–5646. doi:10.1523/JNEUROSCI.4984-12.2013
Author note
R.D. was supported by a doctoral grant from “Studienwerk Villigst.” We thank Julia Preuß, Marie Klippel, Judith Süßenbach, and Carolin Steglich for their help in data acquisition. We assure that we have no financial conflicts of interest regarding the presented work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 398 kb)
Rights and permissions
About this article
Cite this article
Dieterich, R., Endrass, T. & Kathmann, N. Uncertainty is associated with increased selective attention and sustained stimulus processing. Cogn Affect Behav Neurosci 16, 447–456 (2016). https://doi.org/10.3758/s13415-016-0405-8
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-016-0405-8