Published online Mar 26, 2015. doi: 10.4252/wjsc.v7.i2.399
Peer-review started: July 19, 2014
First decision: September 16, 2014
Revised: October 22, 2014
Accepted: November 7, 2014
Article in press: November 10, 2014
Published online: March 26, 2015
Tissue engineering is an emerging field of science that focuses on creating suitable conditions for the regeneration of tissues. The basic components for tissue engineering involve an interactive triad of scaffolds, signaling molecules, and cells. In this context, stem cells (SCs) present the characteristics of self-renewal and differentiation capacity, which make them promising candidates for tissue engineering. Although they present some common markers, such as cluster of differentiation (CD)105, CD146 and STRO-1, SCs derived from various tissues have different patterns in relation to proliferation, clonogenicity, and differentiation abilities in vitro and in vivo. Tooth-derived tissues have been proposed as an accessible source to obtain SCs with limited morbidity, and various tooth-derived SCs (TDSCs) have been isolated and characterized, such as dental pulp SCs, SCs from human exfoliated deciduous teeth, periodontal ligament SCs, dental follicle progenitor cells, SCs from apical papilla, and periodontal ligament of deciduous teeth SCs. However, heterogeneity among these populations has been observed, and the best method to select the most appropriate TDSCs for regeneration approaches has not yet been established. The objective of this review is to outline the current knowledge concerning the various types of TDSCs, and discuss the perspectives for their use in regenerative approaches.
Core tip: Stem cells (SCs) present the characteristics of self-renewal and differentiation capacity, which make them promising candidates for regenerative approaches. Although they present some common markers, SCs derived from various tissues have different patterns of proliferation, clonogenicity, and differentiation. Tooth-derived tissues are an accessible source of SCs with limited morbidity. However, heterogeneity within populations of tooth-derived SCs has been observed, and the best method to select the most appropriate SCs for regenerative approaches has not yet been established.
- Citation: Saito MT, Silvério KG, Casati MZ, Sallum EA, Jr FHN. Tooth-derived stem cells: Update and perspectives. World J Stem Cells 2015; 7(2): 399-407
- URL: https://www.wjgnet.com/1948-0210/full/v7/i2/399.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i2.399
Stem cells (SCs) are cells that present two distinctive characteristics: they are able to continuously self-renew, and they can be induced to differentiate into multiple specialized cell types[1]. SCs have therefore been a subject of interest to researchers and the general public, as a way to regenerate damaged tissues and improve the resolution of some illnesses, such as Parkinson’s disease[2] and diabetes[3], that current approaches in the medical field have not yet achieved. In this context, many studies have been conducted to identify and isolate SCs and to understand their biologic aspects.
SCs can be isolated in the earliest stages of embryogenesis (embryonic SCs)[4-9] or in various postnatal tissues (adult SCs)[2,10,11] (Table 1). Although embryonic SCs present interesting properties, such as the ability to differentiate into hundreds of other cell types, the bioethical aspects involved in the study of these cells, especially for human embryos, have hindered advances in this field and research has thus been focused on adult SCs[1,10-12]. Adult SCs can be obtained from adult specialized tissues, such as bone marrow[13-16], skin[17,18] and fat[19-23], where they likely act to renew cell populations and maintain tissue homeostasis, or help to repair the tissue in case of injury[18,24,25]. Even though adult SCs can be obtained from less ethically concerning sources, they have some limitations compared to embryonic SCs, such as more limited lifespan and differentiation potential[1,11,24,26]. In order to overcome these drawbacks, adult SCs can be reprogrammed by the insertion of SC-associated genes, forming induced pluripotent SCs (iPSCs)[3,27-31].
| Stem cell type | Description |
| Totipotent | Stem cells able to differentiate into cells of all three germ layers (ectoderm, mesoderm and endoderm) and extra-embryonic tissues (e.g., zygote) |
| Pluripotent | Stem cells able to differentiate into all cells of the body, but that cannot form extra-embryonic tissues (e.g., embryonic stem cells and induced pluripotent stem cells) |
| Multipotent | Stem cells that have differentiation abilities restricted to some cell types, usually from the germ layer they are derived from (e.g., mesenchymal stem cells) |
Within the medical field, mesenchymal SCs (MSCs) have been widely studied to understand their role in skeletal tissue development, physiology and repair[14], and because of their promising therapeutic potential[2]. MSCs are characterized by the capacity to differentiate into multiple types of skeletal tissues[14,32-36]. They were first described as adherent, clonogenic, self-renewing, fibroblast-like cells (colony-forming unit fibroblasts) obtained from bone marrow[35,37,38]. Subsequently, several studies were performed to identify other sources and to understand how these cells can give rise to distinct cell types, for the purpose of using these cells in regenerative procedures[39-43].
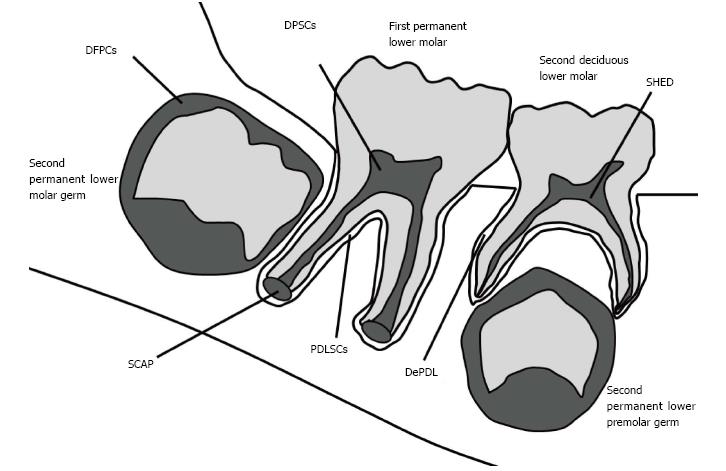
In this context, dental tissues have also been investigated as niches of MSCs, and many tooth-derived SCs (TDSCs) have been identified and characterized, including dental pulp SCs (DPSCs)[44-48], SCs from human exfoliated deciduous teeth (SHED)[49-53], periodontal ligament SCs (PDLSCs), dental follicle progenitor cells (DFPCs)[54-56], SCs from apical papilla (SCAP)[19,56-59], and periodontal ligament of deciduous teeth SCs (DePDL)[50,51,60-62] (Figure 1). Dental tissues are an accessible source of MSCs that can be obtained with limited morbidity and without additional risks to the donor, as extracted/exfoliated teeth represent a waste product of dental procedures[13,63-65]. However, the properties of these TDSCs and their feasibility for regenerating tissues still need to be investigated in greater detail. Thus, the aim of the present review is to describe the current knowledge concerning TDSCs, and to consider the perspectives for their use in regenerative approaches.
Because of the variety of methodologies used to isolate and characterize MSCs, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy proposed minimal criteria to define human bone marrow SCs (BMSCs) and other types of MSCs in vitro[32]. Briefly, MSCs must adhere to plastic under standard culture conditions, express cluster of differentiation (CD)105, CD73 and CD90, but not CD45, CD34, CD14, CD11b, CD79a, CD19, or human leukocyte antigen-DR surface molecules, and have the potential to differentiate along osteogenic, chondrogenic and adipogenic lineages[32]. Therefore, studies characterizing TDSCs usually evaluate these criteria, as well as clonogenicity (capacity to form adherent colonies derived from one single cell) and differentiation potency, in order to compare them to each other and to BMSCs[66,67] (Table 2).
| TDSCs | Ref. | Location | Expression markers | Differentiation capacity | ||
| Positive | Negative | In vitro | In vivo | |||
| DPSCs | [13,44,46,51,65] | Permanent tooth pulp | CD29, CD44, CD73, CD90, CD105, CD146, STRO-1, Oct-3/4, Sox-2, nanog | CD14, CD34, CD45 | Osteoblast, adipocyte, chondrocyte, hepatocyte, neuron | Dentin-like structures |
| SHED | [49,51,53,69,70] | Deciduous tooth pulp | CD29, CD105, CD146, STRO-1 | CD31, CD34 | Osteoblast, odontoblast, adipocyte, neural cell | Dentin formation, induce bone formation by murine host cells |
| SCAP | [13,56,57,65] | Apical papilla of developing tooth | CD24, CD29, CD31, CD44, CD73, CD90, CD105, CD106, CD146, CD166, STRO-1, Oct-3/4, Sox-2, nanog, survivin | CD14, CD18, CD34, CD45, CD150 | Osteoblast, adipocyte, chondrocyte, hepatocyte, neuron | Dentin-like tissue |
| DFPCs | [13,54,56,65,72] | Dental follicle of developing tooth | CD29, CD44, CD73, CD90, CD105, nestin | CD14, CD31, CD34, CD45, CD117 | Osteoblast, adipocyte, chondrocyte, hepatocyte, neuron | Bone/cementum-like tissue |
| PDLSCs | [13,57,60,73] | Permanent tooth periodontal ligament | CD44, CD90, CD105, CD166, CD146, STRO-1, Oct-3/4, Sox2, nanog, nestin | CD14, CD34, CD34, CD45 | Osteoblast/cementoblast,adipocyte, neuron | Periodontal ligament/ cementum-like tissue |
| DePDL | [60] | Deciduous tooth periodontal ligament | CD105, CD166, STRO-Oct-4 | CD34, CD45 | Osteoblast, adipocyte | |
DPSCs were the first TDSCs isolated and characterized in 2000[44]. Obtained from permanent third molars, these cells were found to be more proliferative than BMSCs, and had the capacity to form mineral deposits in vitro, though in reduced amounts compared to BMSCs[44]. DPSCs also failed to form lipid-laden adipocytes in vitro, whereas BMSCs are capable of differentiating into adipocytes[44]. However, more recent studies demonstrate that DPSCs can differentiate into adipocyte cells when other supplements are added to the adipogenic induction medium[46,65].
When transplanted in vivo, some DPSC clones differentiate into aligned odontoblast-like cells, with prolonged processes oriented into newly formed dentin-like structures[44,46], whereas BMSCs form distinct lamellae of bone[44]. DPSCs can also form reparative dentin-like tissue on the surface of human dentin in vivo[68].
In 2003, progenitor cells were isolated from the remnant pulp of exfoliated deciduous teeth[49]. SHED were found to be more proliferative than BMSCs and DPSCs[49,51], and showed higher capability for osteogenic and adipogenic differentiation than DPSCs in vitro[51]. SHED can also differentiate into neural cells[69]. When 12 single-colony-derived SHED clones were transplanted into immunocompromised mice, only three clones demonstrated the potential to generate ectopic dentin-like tissue on the hydroxyapatite/tricalcium phosphate (HA/TCP) carrier equivalent to that generated by multicolony-derived SHED[49]. When SHED were seeded into human tooth slices and transplanted into immunodeficient mice, they were also able to form a dentin-like structure[70]. Although some researchers claim that SHED have the ability to differentiate into osteoblasts in vivo[51], Miura et al[49] reported that, in fact, SHED act as an osteoinductive factor, inducing the host cells to form bone.
The apical papilla is the tissue located at the apex of the root of developing teeth[66], and is distinct from the pulp[71]. As this tissue is associated with root formation, it potentially provides a source of MSCs for this purpose. SCAP are the cells isolated from this tissue that present characteristics of MSCs, and can give rise to odontoblastic, osteoblastic and adipocyte-like cells when cultured under appropriate conditions[57]. SCAP also have the capability to form a dentin-like structure when transplanted into immunocompromised mice, using HA/TCP as a scaffold[57]. Sonoyama et al[57] evaluated whether SCAP and DPSCs are the same or distinct MSC populations based on their cDNA microarray profile, and observed that many genes were differentially expressed by these MSC populations. In particular, CD24 and survivin were highly expressed in the SCAP population. Additionally, SCAP also showed other favorable characteristics, such as higher proliferative rate and telomerase activity, and improved migration capacity[57].
The dental follicle is a condensation of ectomesenchymal cells that surrounds the tooth germ in early stages of tooth formation and contains cells that form the three tissues that constitute the periodontium: periodontal ligament, cementum and alveolar bone[54,72]. When the heterogeneity of DPFCs was analyzed, it was observed that, although all cloned cell lines were positive for MSC-related surface markers (CD105, CD44, CD29) and negative for hematopoietic markers (CD34, CD117), they were different in terms of proliferation and mineralization patterns, indicating that they could be committed to distinct lineages[72].
In order to avoid donor variability, TDSCs from follicle, pulp and papilla were isolated from a single donor tooth and the morphology, proliferation rate, expression of MSC-specific and pluripotency markers, and in vitro differentiation into osteoblasts, adipocytes, chondrocytes and hepatocyte-like cells were compared[65]. Adherent, fibroblast-like morphology was observed in all TDSCs cultured under the same standard conditions, and DFPCs were more proliferative than DPSCs and SCAP[65]. Although all three cell types were able to differentiate into the osteoblast lineage, DFPCs and DPSCs showed higher potentials than SCAP to form mineralized nodules in vitro[65]. Additionally, when cultivated under chondrogenic-inducing conditions, DFPCs expressed all three chondrogenic-specific markers (aggrecan, and type I and type III collagen), whereas DPSCs and SCAP only expressed aggrecan[65].
The periodontal ligament harbors a heterogeneous cell population, with subsets of cells in various stages of commitment to fibroblastic and osteoblastic/cementoblastic lineages[34,73,74]. Within these subsets, it was supposed that putative MSCs would be present in the periodontal ligament, which was confirmed in 2004[73]. PDLSCs show expression of STRO-1 and CD146, SC markers previously reported to be expressed in BMSCs and DPSCs, and also express scleraxis, a tendon-specific transcription factor[73].
PDLSCs are more proliferative and clonogenic than BMSCs[34]. PDLSCs can also differentiate into adipocytes[34,73] and chondrocytes[34], as well as osteoblasts/cementoblasts[34,60,73]. Although PDLSCs are able to form mineralized tissue in vitro when osteoblastic/cementoblastic differentiation is induced, they form fewer mineralized nodules than BMSCs[34,73]. Additionally, when transplanted into immunocompromised mice, some clones of PDLSCs have been shown to form periodontal ligament-like structures in vivo[73].
As MSCs can be isolated from the pulp of deciduous teeth, it was thought that the periodontal ligament of deciduous teeth may also harbor MSCs[60]. In 2010, DePDL were isolated and compared with their permanent counterparts and found to be more proliferative than PDLSCs[60]. Moreover, it was observed that, although DePDL and PDSLCs have the ability to differentiate into both adipocyte-like and osteoblast-like cells in vitro, DePDL show a higher potential for adipogenic commitment, and PDLSCs have a higher potential for osteogenic commitment[60].
Following reports of reprogramming of dermal fibroblasts to behave like embryonic SCs[27,28], studies were conducted to evaluate if other cell types could also be reprogrammed, including TDSCs[29,30]. It was reported that human gingival and periodontal ligament fibroblasts[29], SHED, SCAP and DPSCs[30] can be reprogrammed as iPSCs, with formation of teratomas after implantation in immunocompromised mice[29,30].
Tissue engineering is an emerging field based on basic science and engineering technology, designed to create suitable conditions to regenerate damaged tissues[75-78]. The basic components for tissue engineering involve an interactive triad of scaffolds[79-81], signaling molecules[82-84] and cells[24,33,41,85], which play a fundamental role in the regeneration process[24,76,86,87]. Scaffolds serve as a three-dimensional template mimicking the extracellular matrix; signaling molecules enhance this cellular activity by stimulating cells to migrate, proliferate and differentiate; and cells provide the machinery synthesis of the extracellular matrix and tissue regeneration[24,75,76,88,89]. Due to their interesting properties, including self-renewal and differentiation abilities, MSCs are considered important for tissue maintenance and renewal, and, therefore, a promising candidate for tissue engineering[24,90-94].
Some studies demonstrated that cell-based therapies are able to regenerate dental tissues[48,57,70,95-98]. In a study in dogs, complete pulp regeneration was achieved when CD105+ DPSCs with stromal cell-derived factor-1 were transplanted into pulp, and this was not observed when total pulp cells or CD105+ adipose-derived cells were used[48]. Supplementation of guided tissue regeneration with periodontal ligament cells for the treatment of class II and III furcation defects in dogs enhances periodontal regeneration[95,97]. Twelve weeks after PDLSCs with an HA/TCP scaffold were transplanted into periodontal defects in a minipig model, new bone, cementum and periodontal ligament formation was observed[98]. Sonoyama et al[57] also explored the potential of human PDLSCs and SCAP to generate a root-periodontal ligament complex in minipigs. They were able to obtain engineered roots capable of supporting porcelain, though with lower compressive strength. Nakahara[96] reported the formation of root and periodontal ligament in a new culture system using one tooth crown collected from a neonatal mouse, which was referred to as a “test-tube dental implant”. The author stated that cell therapy will be the next generation of dental medicine, but further information regarding human SCs is necessary for safe and reliable clinical applications[96].
In regard to human clinical trials, autologous progenitor cells obtained from periodontal ligament have been used to treat intrabony defects[99]. These progenitor cells were of a later cell lineage with decreased capacity for osteogenic and adipogenic differentiation compared to PDLSCs in vitro. Despite this, these progenitors were able to promote improvement in clinical and radiographic parameters. Another study reported the cultivation of periodontal ligament cells on titanium pins that were subsequently implanted in patients and in dogs[91]. Clinical evaluation of the implants placed in the patients showed satisfactory mechanical function, and radiographs revealed bone filling and formation of a lamina-dura around the implants. Additionally, histologic evaluation of the implants placed in dogs revealed a ligament-like formation. Although these studies are not directly related to MSCs, they indicate that cell therapy can be a feasible clinical approach in the near future.
Despite these promising studies in cell-based tissue engineering, it is important to highlight that the best method to select the most appropriate MSC type for regenerating dental tissues is not yet clear. Although BMSCs, DPSCs, SHED, SCAP, DFPCs, PDLSCs, and DePDL present a common marker profile, they differ in their clonogenicity, proliferative ability, and differentiation potential in vitro and in vivo, suggesting that these properties are related to the microenvironments of origin of each cell lineage[13,24,34,44,60,65,67,73]. Additionally, it has been noted that, even in the same population of MSCs, there are heterogeneous cell subpopulations with distinct differentiation potentials[100]. This heterogeneity in relation to the ability to differentiate in vitro and to form dental tissues in vivo has also been reported in some TDSCs lineages, including DPSCs[44,46,67], SHED[49], DFPCs[72], and PDLSCs[34,73,74,101-104]. Therefore, it can be concluded that, although there are MSC-related surface markers, such as STRO-1, CD146 and CD105, specific surface markers associated with the hierarchical commitment to differentiation pathways of TDSCs are not yet well established. In this context, further advances in understanding the regulation of MSCs during differentiation and dental development are required in order to develop new approaches for dental tissue regeneration with predictable outcomes[19,26,67,89].
The interest in organ regeneration using SCs has increased in the last decade. In this context, TDSCs are promising candidates, as they are readily available, highly proliferative, and present multi-differentiation abilities. Research on cell therapy for regenerating dental tissues has already been done, and shows promising results. Nevertheless, further research is needed to better characterize TDSCs and to understand their differentiation pathways in order to develop the most appropriate approaches for SC-based tissue engineering-therapies in dental practice.
P- Reviewer: Aponte PM, Foss B, Roelen B, Song SU S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Fischbach GD, Fischbach RL. Stem cells: science, policy, and ethics. J Clin Invest. 2004;114:1364-1370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Watt FM, Driskell RR. The therapeutic potential of stem cells. Philos Trans R Soc Lond B Biol Sci. 2010;365:155-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Vogel G. Stem cells. Therapeutic cloning reaches milestone. Science. 2014;344:462-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, An Z, Wang L, Hultman I, Ahrlund-Richter L. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 2014;513:551-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 284] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 5. | Gothard D, Roberts SJ, Shakesheff KM, Buttery LD. Engineering embryonic stem-cell aggregation allows an enhanced osteogenic differentiation in vitro. Tissue Eng Part C Methods. 2010;16:583-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Lock LT, Tzanakakis ES. Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture. Tissue Eng Part A. 2009;15:2051-2063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Wang X, Ye K. Three-dimensional differentiation of embryonic stem cells into islet-like insulin-producing clusters. Tissue Eng Part A. 2009;15:1941-1952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Hillel AT, Varghese S, Petsche J, Shamblott MJ, Elisseeff JH. Embryonic germ cells are capable of adipogenic differentiation in vitro and in vivo. Tissue Eng Part A. 2009;15:479-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Inanç B, Elçin AE, Elçin YM. Human embryonic stem cell differentiation on tissue engineering scaffolds: effects of NGF and retinoic acid induction. Tissue Eng Part A. 2008;14:955-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Hyun I. The bioethics of stem cell research and therapy. J Clin Invest. 2010;120:71-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Hviid Nielsen T. What happened to the stem cells? J Med Ethics. 2008;34:852-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | de Vries RB, Oerlemans A, Trommelmans L, Dierickx K, Gordijn B. Ethical aspects of tissue engineering: a review. Tissue Eng Part B Rev. 2008;14:367-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Tamaki Y, Nakahara T, Ishikawa H, Sato S. In vitro analysis of mesenchymal stem cells derived from human teeth and bone marrow. Odontology. 2013;101:121-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3539] [Cited by in F6Publishing: 3155] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 15. | Menicanin D, Bartold PM, Zannettino AC, Gronthos S. Identification of a common gene expression signature associated with immature clonal mesenchymal cell populations derived from bone marrow and dental tissues. Stem Cells Dev. 2010;19:1501-1510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | D’Angelo F, Armentano I, Cacciotti I, Tiribuzi R, Quattrocelli M, Del Gaudio C, Fortunati E, Saino E, Caraffa A, Cerulli GG. Tuning multi/pluri-potent stem cell fate by electrospun poly(L-lactic acid)-calcium-deficient hydroxyapatite nanocomposite mats. Biomacromolecules. 2012;13:1350-1360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Voigt M, Schauer M, Schaefer DJ, Andree C, Horch R, Stark GB. Cultured epidermal keratinocytes on a microspherical transport system are feasible to reconstitute the epidermis in full-thickness wounds. Tissue Eng. 1999;5:563-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Blanpain C, Fuchs E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science. 2014;344:1242281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 377] [Cited by in F6Publishing: 394] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 19. | Huang GT, Al-Habib M, Gauthier P. Challenges of stem cell-based pulp and dentin regeneration: a clinical perspective. Endod Topics. 2013;28:51-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Rada T, Reis RL, Gomes ME. Distinct stem cells subpopulations isolated from human adipose tissue exhibit different chondrogenic and osteogenic differentiation potential. Stem Cell Rev. 2011;7:64-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Okura H, Komoda H, Saga A, Kakuta-Yamamoto A, Hamada Y, Fumimoto Y, Lee CM, Ichinose A, Sawa Y, Matsuyama A. Properties of hepatocyte-like cell clusters from human adipose tissue-derived mesenchymal stem cells. Tissue Eng Part C Methods. 2010;16:761-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Hildner F, Concaro S, Peterbauer A, Wolbank S, Danzer M, Lindahl A, Gatenholm P, Redl H, van Griensven M. Human adipose-derived stem cells contribute to chondrogenesis in coculture with human articular chondrocytes. Tissue Eng Part A. 2009;15:3961-3969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Tsuji W, Rubin JP, Marra KG. Adipose-derived stem cells: Implications in tissue regeneration. World J Stem Cells. 2014;6:312-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 219] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 24. | Han J, Menicanin D, Gronthos S, Bartold PM. Stem cells, tissue engineering and periodontal regeneration. Aust Dent J. 2014;59 Suppl 1:117-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 25. | Tolar J, Le Blanc K, Keating A, Blazar BR. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446-1455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 307] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 26. | Silvério KG, Benatti BB, Casati MZ, Sallum EA, Nociti FH. Stem cells: potential therapeutics for periodontal regeneration. Stem Cell Rev. 2008;4:13-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17989] [Cited by in F6Publishing: 17055] [Article Influence: 947.5] [Reference Citation Analysis (0)] |
| 28. | Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2182] [Cited by in F6Publishing: 2075] [Article Influence: 122.1] [Reference Citation Analysis (0)] |
| 29. | Wada N, Wang B, Lin NH, Laslett AL, Gronthos S, Bartold PM. Induced pluripotent stem cell lines derived from human gingival fibroblasts and periodontal ligament fibroblasts. J Periodontal Res. 2011;46:438-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Yan X, Qin H, Qu C, Tuan RS, Shi S, Huang GT. iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cells Dev. 2010;19:469-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 31. | Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24:2239-2263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 573] [Cited by in F6Publishing: 540] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 32. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11055] [Cited by in F6Publishing: 11776] [Article Influence: 692.7] [Reference Citation Analysis (1)] |
| 33. | P M, S H, R M, M G, W S K. Adult mesenchymal stem cells and cell surface characterization - a systematic review of the literature. Open Orthop J. 2011;5:253-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 34. | Gay IC, Chen S, MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res. 2007;10:149-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 281] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 35. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15372] [Cited by in F6Publishing: 14773] [Article Influence: 590.9] [Reference Citation Analysis (0)] |
| 36. | Greco SJ, Liu K, Rameshwar P. Functional similarities among genes regulated by OCT4 in human mesenchymal and embryonic stem cells. Stem Cells. 2007;25:3143-3154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 37. | Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267-274. [PubMed] [Cited in This Article: ] |
| 38. | Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263-272. [PubMed] [Cited in This Article: ] |
| 39. | Jurga M, Lipkowski AW, Lukomska B, Buzanska L, Kurzepa K, Sobanski T, Habich A, Coecke S, Gajkowska B, Domanska-Janik K. Generation of functional neural artificial tissue from human umbilical cord blood stem cells. Tissue Eng Part C Methods. 2009;15:365-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | He F, Chen X, Pei M. Reconstruction of an in vitro tissue-specific microenvironment to rejuvenate synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2009;15:3809-3821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 41. | Reinke S, Dienelt A, Blankenstein A, Duda GN, Geissler S. Qualifying stem cell sources: how to overcome potential pitfalls in regenerative medicine? J Tissue Eng Regen Med. 2014;Jun 12; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Olson HE, Rooney GE, Gross L, Nesbitt JJ, Galvin KE, Knight A, Chen B, Yaszemski MJ, Windebank AJ. Neural stem cell- and Schwann cell-loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord. Tissue Eng Part A. 2009;15:1797-1805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 43. | Vellasamy S, Sandrasaigaran P, Vidyadaran S, George E, Ramasamy R. Isolation and characterisation of mesenchymal stem cells derived from human placenta tissue. World J Stem Cells. 2012;4:53-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 44. | Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625-13630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3174] [Cited by in F6Publishing: 3128] [Article Influence: 130.3] [Reference Citation Analysis (0)] |
| 45. | Yang X, Walboomers XF, van den Beucken JJ, Bian Z, Fan M, Jansen JA. Hard tissue formation of STRO-1-selected rat dental pulp stem cells in vivo. Tissue Eng Part A. 2009;15:367-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 46. | Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1439] [Cited by in F6Publishing: 1334] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 47. | Zhang W, Walboomers XF, Wolke JG, Bian Z, Fan MW, Jansen JA. Differentiation ability of rat postnatal dental pulp cells in vitro. Tissue Eng. 2005;11:357-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Iohara K, Imabayashi K, Ishizaka R, Watanabe A, Nabekura J, Ito M, Matsushita K, Nakamura H, Nakashima M. Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng Part A. 2011;17:1911-1920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 49. | Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807-5812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1922] [Cited by in F6Publishing: 1852] [Article Influence: 88.2] [Reference Citation Analysis (0)] |
| 50. | Fu X, Jin L, Ma P, Fan Z, Wang S. Allogeneic stem cells from deciduous teeth in treatment for periodontitis in miniature swine. J Periodontol. 2014;85:845-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 51. | Wang X, Sha XJ, Li GH, Yang FS, Ji K, Wen LY, Liu SY, Chen L, Ding Y, Xuan K. Comparative characterization of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. Arch Oral Biol. 2012;57:1231-1240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 52. | Zheng Y, Liu Y, Zhang CM, Zhang HY, Li WH, Shi S, Le AD, Wang SL. Stem cells from deciduous tooth repair mandibular defect in swine. J Dent Res. 2009;88:249-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 53. | Behnia A, Haghighat A, Talebi A, Nourbakhsh N, Heidari F. Transplantation of stem cells from human exfoliated deciduous teeth for bone regeneration in the dog mandibular defect. World J Stem Cells. 2014;6:505-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kühn U, Möhl C, Sippel C, Hoffmann KH. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 609] [Cited by in F6Publishing: 581] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 55. | Silvério KG, Davidson KC, James RG, Adams AM, Foster BL, Nociti FH, Somerman MJ, Moon RT. Wnt/β-catenin pathway regulates bone morphogenetic protein (BMP2)-mediated differentiation of dental follicle cells. J Periodontal Res. 2012;47:309-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Guo L, Li J, Qiao X, Yu M, Tang W, Wang H, Guo W, Tian W. Comparison of odontogenic differentiation of human dental follicle cells and human dental papilla cells. PLoS One. 2013;8:e62332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 835] [Cited by in F6Publishing: 830] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 58. | Zhang W, Zhang X, Ling J, Liu W, Zhang X, Ma J, Zheng J. Proliferation and odontogenic differentiation of BMP2 gene-transfected stem cells from human tooth apical papilla: an in vitro study. Int J Mol Med. 2014;34:1004-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 59. | Wang J, Liu B, Gu S, Liang J. Effects of Wnt/β-catenin signalling on proliferation and differentiation of apical papilla stem cells. Cell Prolif. 2012;45:121-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 60. | Silvério KG, Rodrigues TL, Coletta RD, Benevides L, Da Silva JS, Casati MZ, Sallum EA, Nociti FH. Mesenchymal stem cell properties of periodontal ligament cells from deciduous and permanent teeth. J Periodontol. 2010;81:1207-1215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 61. | Fukushima H, Kawanabe N, Murata S, Ishihara Y, Yanagita T, Balam TA, Yamashiro T. SSEA-4 is a marker of human deciduous periodontal ligament stem cells. J Dent Res. 2012;91:955-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Ji K, Liu Y, Lu W, Yang F, Yu J, Wang X, Ma Q, Yang Z, Wen L, Xuan K. Periodontal tissue engineering with stem cells from the periodontal ligament of human retained deciduous teeth. J Periodontal Res. 2013;48:105-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Chen FM, Sun HH, Lu H, Yu Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials. 2012;33:6320-6344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 64. | Morad G, Kheiri L, Khojasteh A. Dental pulp stem cells for in vivo bone regeneration: a systematic review of literature. Arch Oral Biol. 2013;58:1818-1827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 65. | Patil R, Kumar BM, Lee WJ, Jeon RH, Jang SJ, Lee YM, Park BW, Byun JH, Ahn CS, Kim JW. Multilineage potential and proteomic profiling of human dental stem cells derived from a single donor. Exp Cell Res. 2014;320:92-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 66. | Sedgley CM, Botero TM. Dental stem cells and their sources. Dent Clin North Am. 2012;56:549-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 67. | Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1184] [Cited by in F6Publishing: 1215] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 68. | Batouli S, Miura M, Brahim J, Tsutsui TW, Fisher LW, Gronthos S, Robey PG, Shi S. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res. 2003;82:976-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 307] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 69. | Nourbakhsh N, Soleimani M, Taghipour Z, Karbalaie K, Mousavi SB, Talebi A, Nadali F, Tanhaei S, Kiyani GA, Nematollahi M. Induced in vitro differentiation of neural-like cells from human exfoliated deciduous teeth-derived stem cells. Int J Dev Biol. 2011;55:189-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 70. | Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nör JE. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 430] [Cited by in F6Publishing: 459] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 71. | Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 745] [Cited by in F6Publishing: 740] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 72. | Luan X, Ito Y, Dangaria S, Diekwisch TG. Dental follicle progenitor cell heterogeneity in the developing mouse periodontium. Stem Cells Dev. 2006;15:595-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 73. | Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2371] [Cited by in F6Publishing: 2336] [Article Influence: 116.8] [Reference Citation Analysis (0)] |
| 74. | Saito MT, Salmon CR, Amorim BR, Ambrosano GM, Casati MZ, Sallum EA, Nociti FH, Silvério KG. Characterization of highly osteoblast/cementoblast cell clones from a CD105-enriched periodontal ligament progenitor cell population. J Periodontol. 2014;85:e205-e211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 75. | Scheller EL, Krebsbach PH, Kohn DH. Tissue engineering: state of the art in oral rehabilitation. J Oral Rehabil. 2009;36:368-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 76. | Bartold PM, McCulloch CA, Narayanan AS, Pitaru S. Tissue engineering: a new paradigm for periodontal regeneration based on molecular and cell biology. Periodontol 2000. 2000;24:253-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 282] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 77. | Slavkin HC, Bartold PM. Challenges and potential in tissue engineering. Periodontol 2000. 2006;41:9-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Benatti BB, Silvério KG, Casati MZ, Sallum EA, Nociti FH. Physiological features of periodontal regeneration and approaches for periodontal tissue engineering utilizing periodontal ligament cells. J Biosci Bioeng. 2007;103:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Liao F, Chen Y, Li Z, Wang Y, Shi B, Gong Z, Cheng X. A novel bioactive three-dimensional beta-tricalcium phosphate/chitosan scaffold for periodontal tissue engineering. J Mater Sci Mater Med. 2010;21:489-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 80. | Cunniffe GM, Dickson GR, Partap S, Stanton KT, O’Brien FJ. Development and characterisation of a collagen nano-hydroxyapatite composite scaffold for bone tissue engineering. J Mater Sci Mater Med. 2010;21:2293-2298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 81. | Ahn S, Yoon H, Kim G, Kim Y, Lee S, Chun W. Designed three-dimensional collagen scaffolds for skin tissue regeneration. Tissue Eng Part C Methods. 2010;16:813-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 82. | Yang L, Zhang Y, Dong R, Peng L, Liu X, Wang Y, Cheng X. Effects of adenoviral-mediated coexpression of bone morphogenetic protein-7 and insulin-like growth factor-1 on human periodontal ligament cells. J Periodontal Res. 2010;45:532-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 83. | Kao RT, Murakami S, Beirne OR. The use of biologic mediators and tissue engineering in dentistry. Periodontol 2000. 2009;50:127-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Saito A, Saito E, Handa R, Honma Y, Kawanami M. Influence of residual bone on recombinant human bone morphogenetic protein-2-induced periodontal regeneration in experimental periodontitis in dogs. J Periodontol. 2009;80:961-968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 85. | Egusa H, Sonoyama W, Nishimura M, Atsuta I, Akiyama K. Stem cells in dentistry--part I: stem cell sources. J Prosthodont Res. 2012;56:151-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 208] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 86. | Zhang Y, Wang Y, Shi B, Cheng X. A platelet-derived growth factor releasing chitosan/coral composite scaffold for periodontal tissue engineering. Biomaterials. 2007;28:1515-1522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 87. | Nakahara T, Nakamura T, Kobayashi E, Inoue M, Shigeno K, Tabata Y, Eto K, Shimizu Y. Novel approach to regeneration of periodontal tissues based on in situ tissue engineering: effects of controlled release of basic fibroblast growth factor from a sandwich membrane. Tissue Eng. 2003;9:153-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 88. | Taba M, Jin Q, Sugai JV, Giannobile WV. Current concepts in periodontal bioengineering. Orthod Craniofac Res. 2005;8:292-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 89. | Feng R, Lengner C. Application of Stem Cell Technology in Dental Regenerative Medicine. Adv Wound Care (New Rochelle). 2013;2:296-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 90. | Egusa H, Sonoyama W, Nishimura M, Atsuta I, Akiyama K. Stem cells in dentistry--Part II: Clinical applications. J Prosthodont Res. 2012;56:229-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 91. | Gault P, Black A, Romette JL, Fuente F, Schroeder K, Thillou F, Brune T, Berdal A, Wurtz T. Tissue-engineered ligament: implant constructs for tooth replacement. J Clin Periodontol. 2010;37:750-758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | Koch L, Kuhn S, Sorg H, Gruene M, Schlie S, Gaebel R, Polchow B, Reimers K, Stoelting S, Ma N. Laser printing of skin cells and human stem cells. Tissue Eng Part C Methods. 2010;16:847-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 322] [Cited by in F6Publishing: 225] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 93. | Lin NH, Gronthos S, Bartold PM. Stem cells and future periodontal regeneration. Periodontol 2000. 2009;51:239-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 94. | Geuze RE, Wegman F, Oner FC, Dhert WJ, Alblas J. Influence of endothelial progenitor cells and platelet gel on tissue-engineered bone ectopically in goats. Tissue Eng Part A. 2009;15:3669-3677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 95. | Suaid FF, Ribeiro FV, Rodrigues TL, Silvério KG, Carvalho MD, Nociti FH, Casati MZ, Sallum EA. Autologous periodontal ligament cells in the treatment of class II furcation defects: a study in dogs. J Clin Periodontol. 2011;38:491-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 96. | Nakahara T. Potential feasibility of dental stem cells for regenerative therapies: stem cell transplantation and whole-tooth engineering. Odontology. 2011;99:105-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 97. | Suaid FF, Ribeiro FV, Gomes TR, Silvério KG, Carvalho MD, Nociti FH, Casati MZ, Sallum EA. Autologous periodontal ligament cells in the treatment of Class III furcation defects: a study in dogs. J Clin Periodontol. 2012;39:377-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 98. | Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, Gronthos S, Shi S, Wang S. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells. 2008;26:1065-1073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 412] [Cited by in F6Publishing: 407] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 99. | Feng F, Akiyama K, Liu Y, Yamaza T, Wang TM, Chen JH, Wang BB, Huang GT, Wang S, Shi S. Utility of PDL progenitors for in vivo tissue regeneration: a report of 3 cases. Oral Dis. 2010;16:20-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 100. | Okamoto T, Aoyama T, Nakayama T, Nakamata T, Hosaka T, Nishijo K, Nakamura T, Kiyono T, Toguchida J. Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. Biochem Biophys Res Commun. 2002;295:354-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 202] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 101. | Singhatanadgit W, Donos N, Olsen I. Isolation and characterization of stem cell clones from adult human ligament. Tissue Eng Part A. 2009;15:2625-2636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Fujii S, Maeda H, Wada N, Tomokiyo A, Saito M, Akamine A. Investigating a clonal human periodontal ligament progenitor/stem cell line in vitro and in vivo. J Cell Physiol. 2008;215:743-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 103. | Wang L, Shen H, Zheng W, Tang L, Yang Z, Gao Y, Yang Q, Wang C, Duan Y, Jin Y. Characterization of stem cells from alveolar periodontal ligament. Tissue Eng Part A. 2011;17:1015-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 104. | Sununliganon L, Singhatanadgit W. Highly osteogenic PDL stem cell clones specifically express elevated levels of ICAM1, ITGB1 and TERT. Cytotechnology. 2012;64:53-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |