Abstract
Composite coatings were prepared by using epoxy-hardener having a 2:1 composition and functionalized SiO2 having 0, 0.01, 0.03, and 0.05 proportion/s accordingly. Curing of the coatings on aluminium alloy 7075 substrates was performed at a temperature of 60 ± 5 °C. Moreover, corrosion assessment was carried out in 0.6M NaCl solution at room temperature. The outcome of Fourier Transform Infrared Spectroscopy (FT-IR) confirmed the presence of Si-O bond and epoxide peaks. XRD spectra depict that the intensity of AA-7075 peaks was substantially effected possibly due to more x-rays absorption in composite coatings as a result of increasing silica content. Electrochemical Impedance Spectroscopy (EIS) analysis depicted 116, 284, 620, and 1309% increase in charge transfer resistance (Rct) for 0, 1, 3, and 5% silica composition relative to bare AA-7075 samples. Moreover, potentiodynamic plots were used to estimate corrosion rate which depicted decline of 20, 55, 40, 90 and 97% when compared with pure AA-7075 substrate.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Aluminium alloys have various applications and are widely used as a structural material due to their high strength-to-weight and the high strength-to-volume ratio [1]. Numerous types of aluminium alloys with different compositions and then performing heat treatment make them suitable for the manufacturing of various products. Examples are aluminium alloys of series 5xxx, 6xxx, and 7xxx have excellent mechanical properties. They are normally used in aerospace, marine, sports items, and other structural applications. Unluckily, their corrosion resistance is low for some applications. In order to protect it, surface coatings are needed [2]. Researchers have evaluated several aspects of aluminium alloys and their possible synthesized coating materials having diverse chemistry. Epoxy belongs to the group of versatile polymers used in various engineering applications. Epoxy resin was initially synthesized in 1930. Numerous types of epoxy resins and their curing agents have been manufactured with the passage of time. Epoxy resins are widely utilized as coating, potting and adhesive material in aerospace, industrial tooling, marine, and in the fabrication of different electronic components in the electronic industry. Some specific epoxy resins having resistance to ultraviolet light are extensively used in the field of fiber optics, optoelectronics, and dentistry. The epoxide group is a three-membered ring containing one oxygen and two carbon atoms, also known as ethoxyline or oxirane group. Due to this special structure, it takes part very easily in addition polymerization by opening the molecular ring. The properties of epoxy-based coatings have main dependency on the overall molecular structure, amount of the curing agent used, and curing conditions [3, 4]. Due to its excellent properties, epoxy resin is used in various polymeric applications. Epoxies have high adhesion power with other materials, lower curing temperature, good moisture resistance, and excellent mechanical properties such as good impact resistance, high stiffness, and strength [5, 6]. Epoxy resin and amine composite coatings are preferred to use for protection in corrosive environments [7]. It requires a complete understanding of the relationship between the structure and property of the material in order to develop better coatings having improved properties [8]. There are two aspects on which the epoxies are modified. First to develop and modify the epoxy resin from the synthesis aspect and second to use the synthesized composite in various applications i.e.; adhesives, varnishes, paints, coatings, and in manufacturing of construction materials and so on [9, 10]. For the corrosion protection of metals and alloys, coatings of epoxy resins are extensively applied. These polymeric coatings act as a barrier between the metallic surfaces and the corrosion environment [11–14].
For improving the durability and performance of coatings, inorganic fillers are added to the polymeric resins. Polymeric composite coatings have gotten immense attention over the last few decades, to combine the advantageous properties of both polymeric resin and fillers in one coating, and are termed high-performance coatings [15–18]. Silica nanoparticles have been investigated for their potential to enhance coatings' anticorrosion and scratch and abrasion resistance [19]. Silane coupling agents (RSiX3), where X stands for any hydrolyzable groups, such as methoxy, ethoxy, or chloro groups, are used to modify inorganic fillers in order to prevent agglomeration [20–22]. For optimal dispersion and mixing in a polymer. Ghanbari et al treated silica particles with 3-Glycidoxypropyltrimethoxysilane. Composite coatings containing modified silica particles have been found to have higher corrosion resistance [23]. Synthesized silica along with polymer was used for coating purposes and it was observed that it enhanced the corrosion resistance of austenitic steel against conductive ions [24]. Silica's impacts on epoxy-coated stainless steel led to a notable improvement against chloride ions which as a consequence decreases the possibility of delamination [25]. Several percentages of pigments were doped into an epoxy-polyamine cross-linking solution before it was applied on the surface of mild steel. As the percentage of lignin-based azo pigment increased, it was seen that the coating's chemical resistance improved [26]. An effort was made to highlight recent developments in the creation of sustainable hybrid nanocomposite coatings for the corrosion prevention of structural metals in a topical review article. With a focus on active corrosion inhibition, the effects of including metallic, porous metal oxide, and carbon nanomaterials (graphene and carbon nanotubes) within polymeric matrices were investigated. Unprecedented functionality and opportunities for multifunctional coatings are made possible by the availability of high-quality nanoparticles that are either electroactive or capable of acting as reservoirs for active corrosion inhibitors, e.g., porous silicon oxide [27]. Graphene oxide and oxidised multiwalled carbon nanotubes were utilised as curing agents in a unique synthetic method to cause the cross-linking of an epoxy resin, producing a nanostructured epoxy composite with good carbon nanomaterial dispersion. The distribution, integrity, and potential to resist corrosion on a steel surface were confirmed by structural, mechanical, and morphological evaluation of the composite material, which also demonstrates the outstanding adherence and flexibility of the nanocomposite coatings [28]. Investigations were made into the corrosion behaviour of hot-dip galvanised steel that had either been prepared with bis-1,2-[triethoxysilyl]ethane silane (BTSE) or bis-[triethoxysilylpropyl] tetrasulfide (BTESPT) modified with SiO2 micro-particles. The outcomes demonstrate that hot-dip galvanised steel is protected from corrosion during submersion in NaCl solution by pretreatments based on silane films modified with micro-silica particles [29]. In order to improve the mechanical and thermal properties of tetrafunctional epoxy nanocomposites, the research explored the synergistic effect of graphene oxide, nano and micro-silica particle composite. A physical barrier created by the hollow micro-silica particles prevents agglomeration and restacking. The amine-functionalized silica particles make it easier for graphene oxide and epoxy to chemically react [30]. The ultrasonic method was used to successfully synthesize blank epoxy coatings (EC) and fumed silica epoxy composite coatings (SECCs) augmented with various loading levels of nano (7 nm) and micro (0.2–0.3 μm) silica. Contact angle measurements suggest that micro-silica treated coatings exhibit improved hydrophobicity in contrast to nano-silica samples [31]. Furthermore, steel specimen substrates were treated with latex. Under marine exposure conditions, it was concluded that coatings comprising latex, micro silica, zinc phosphate, ferric oxide, aluminium oxide, titanium oxide, and silica fume had higher corrosion resistance [32]. Given the gap, the goal of this research work is to produce epoxy-based composite coatings at a curing temperature of 60 ± 5 °C, by incorporating APTES functionalized SiO2 micro-particles having concentrations of 0 × 104, 1 × 104, 3 × 104 and 5 × 104 ppm. The findings of this study offer a useful strategy for increasing adherence and corrosion resistance of the epoxy coatings while reducing hydrophilicity. In the current study, FT-IR and XRD analyses were used to confirm successful integration of SiO2 with epoxy along with composition and molecular structure of composite. Electrochemical tests were conducted in an electrolyte artificial seawater ASTM standard D1141-98 having a molarity of 0.6M, to evaluate the corrosion resistance of composite coatings, which to the best of our knowledge has not been performed in any previous study. Furthermore, the effect of functionalized silica incorporation in coatings has been associated with the electrochemical behavior of the produced composite coatings on aluminium alloy 7075 substrates.
2. Experimental
2.1. Materials
The aluminium alloy AA7075 material was purchased from Soan Enterprises Islamabad, Pakistan. The polymeric epoxy resin (Waterpoxy 1422) having the appearance milky emulsion and amine hardener (Waterpoxy 751) were both purchased from BASF chemicals company, Germany. The filler material silica gel 60 used is of Merck company, having particle size range 63–200 micro-meter and mesh size 70–230. The coupling agent 3-aminopropyltriethoxysilane (APTES) was purchased from Sigma-Aldrich Chemical Co. and used without further purification.
2.2. Composition of AA 7075
The composition of the alloy was investigated by Optical Emission Spectrometry (OES), shown in table 1. The obtained result confirmed the composition of aluminium alloy AA7075.
Table 1. Optical emission spectrometric analysis of AA7075.
| wt% | Si | Fe | Cu | Mn | Mg | Cr | Ni | Zn | Ti | V |
|---|---|---|---|---|---|---|---|---|---|---|
| Min | 0.19 | 0.21 | 1.48 | 0.03 | 2.27 | 0.21 | 0.005 | 5.3 | 0.02 | 0.006 |
| Max | 0.22 | 0.26 | 1.71 | 0.1 | 2.56 | 0.25 | 0.01 | 5.87 | 0.07 | 0.01 |
| Mean | 0.21 | 0.24 | 1.60 | 0.07 | 2.42 | 0.23 | 0.008 | 5.59 | 0.045 | 0.008 |
2.3. Functionalization of micro-silica
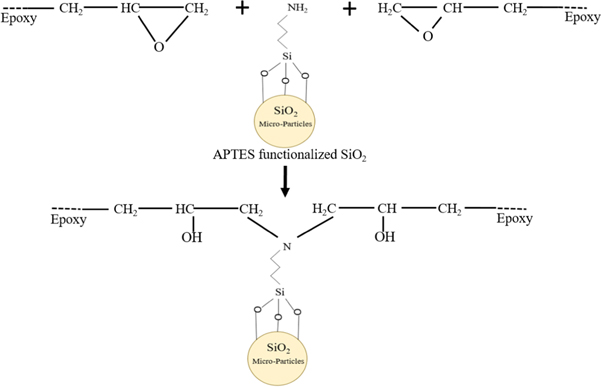
The amine-functionalized micro silica was prepared and subsequently used in the polymeric coatings. In a typical procedure, 0.45 g of silica micro-particles were added slowly and gradually to 100 ml of toluene and stirred properly at a temperature of 60 °C for 2.5 h. Further 1.5 g of 3-aminopropyltriethoxysilane (APTES) was introduced to the flask as a coupling agent. The mixture was then allowed for 20 h to be refluxed. The solid phase was separated by centrifugation and repeatedly washed with methanol and DI water for cleaning and to remove coupling agent residuals if any. After washing, the obtained solid phase was dried in an oven at 80 °C for 20 h. The scheme for the functionalization of SiO2 is depicted in figure 1.
Figure 1. Scheme for functionalization of SiO2 micro-particles with APTES.
Download figure:
Standard image High-resolution image2.4. Synthesis of composite coatings
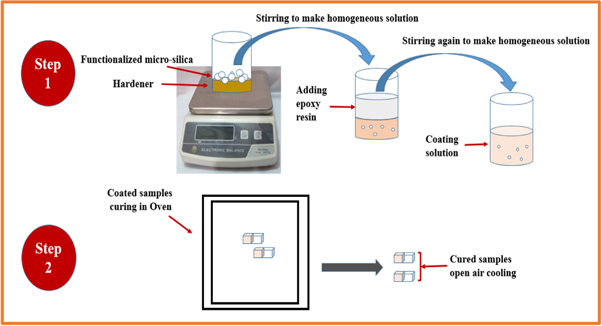
The aluminium alloy AA 7075 having dimensions of 10 mm × 10 mm × 3 mm was used as the substrate surface. The surface of all samples was polished with SiC papers having grit sizes of 800 1200 and 2000. Ethanol was used to perform degreasing and lastly dried in the open air. All composite coatings were fabricated using the epoxy resin and hardener in 2:1.The composite of epoxy-functionalized micro-silica was prepared by putting functionalized silica in 5 g of hardener in a beaker and stirring it mechanically for 10 min at 30 °C. Then 10 g of epoxy resin was added to the mixture and stirred again for 15 min at 35 °C to make a homogenous suspension. The suspension was then applied on the substrate aluminium alloy AA 7075 using dip coating technique, and allowed it to dry for 3 h at room temperature and subsequently cured at 60 °C. The dry coating thickness of the samples was in the range of 120 ± 10 μm. The synthesis of composite coating suspension and its curing on the substrate alloy is shown in figure 2. All the samples were prepared exactly in the same manner. The designation system for samples is shown in table 2.
Figure 2. Synthesis of functionalized SiO2 based solution and its curing at substrate AA 7075.
Download figure:
Standard image High-resolution imageTable 2. Sample/Coatings designation.
| Sample | Description |
|---|---|
| AA 7075 | Aluminium alloy of 7xxx series |
| EHF0 | Epoxy two parts, Hardener one part, Filler zero percent (0 × 104 ppm) |
| EHF1 | Epoxy two parts, Hardener one part, Filler one percent (1 × 104 ppm) |
| EHF3 | Epoxy two parts, Hardener one part, Filler three percent (3 × 104 ppm) |
| EHF5 | Epoxy two parts, Hardener one part, Filler five percent (5 × 104 ppm) |
2.5. Characterizations
Numerous qualitative and quantitative techniques were employed to investigate the composition, phase, morphology, structure, and electrochemical behavior of the fabricated samples. The elemental composition of AA 7075 was determined by using the optical emission spectrometer SPAS-02 Active Co. Ltd The dry coating thickness was measured by using a coating thickness gauge, model DCFN-3000EZ. The chemical composition investigation was carried out over 400–4000 cm−1 wavelength range by Fourier transforms infrared spectroscopy using FT/IR-6600 Spectrometer. The bare aluminium alloy 7075 and coated samples were investigated by using CuKα radiation (λ = 1.54056 A°) of high-a resolution x-rays diffractometer. The electrochemical behavior of samples was assessed with electrochemical workstation, Gammry Potentiostat 1010 by conducting potentiostatic EIS and potentiodynamic tests.
2.6. Electrochemical tests
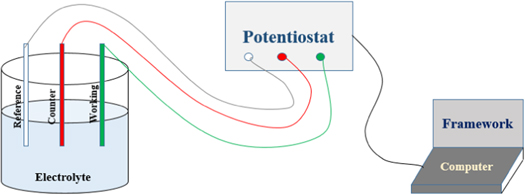
The electrochemical measurements were performed in this research including electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization (PDP).The electrochemical study of the samples conducted using Gamry potentiostat system. The experiments were executed in electrochemical cell having three electrodes, consist of saturated Ag/AgCl reference electrode, platinum counter electrode and aluminium alloy 7075 substrate as working electrode. The three electrodes setup used, to get the data from the various electrochemical tests as shown in figure 3. The working electrode exposed area was 3 cm2. The electrolytic solution used was 0.6 M or 3.5% NaCl solution (artificial seawater ASTM standard D1141–98). Every time for each set of experiments fresh solution was used. The EIS plots were obtained for the frequency range of 100000–0.1 Hz. Furthermore, the Tafel curves were plotted in the electric potential range (−0.1 to 1 V). All the measurements were carried out at room temperature. For all measurements, the tests were repeated at least three times for reproduction of the results and evaluation of the used experimental setup.
Figure 3. Schematic diagram of three electrodes based electrochemical setup.
Download figure:
Standard image High-resolution image3. Results and discussion
3.1. Reaction mechanism
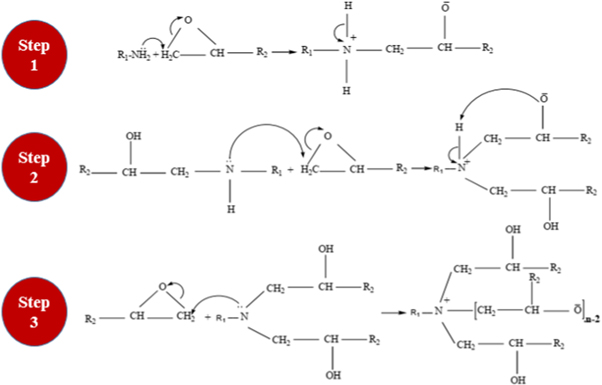
The 3-aminopropyltriethoxysilane (APTES) was used as a coupling agent in the preparation of aminated organo micro-silica particles. The coupling agent APTES is effective in making a layer on the surface of micro-silica and enhances the particles' dispersion and wettability [33]. In the curing of epoxy resin, the amino groups present on the surface of silica micro-particles provide an opportunity to act as a co-hardener. The main reaction occurs between the curing agent and epoxy resin. The reaction between epoxy and amine starts when primary amine attacks the epoxide group of epoxy and opens it in the first step. In the second step, the secondary amine produced from the first step attacks another epoxide group and opens it. The tertiary amine produced as a result of the second step again attacks the epoxide group in the third step. Due to the reaction of tertiary amine and epoxide group a reaction occurs which is normally termed as etherification. The reaction of etherification might take place once amine hydrogen atoms deplete and if epoxide groups are available in extra. The reaction mechanism is depicted in three steps as shown in figure 4.
Figure 4. Reaction mechanism of hardener with epoxy resin.
Download figure:
Standard image High-resolution imageHowever, there is a reaction between functionalized micro-silica particles and epoxy resin as well as presented in figure 5. In the coating synthesis, amine hardener was employed as a curing agent. The mobility of the molecules within the system progressively lessens as the reaction continues to form a network structure. After curing the epoxy-hardener liquid mixture, an extremely cross-linked three-dimensional network is formed.
Figure 5. Reaction between functionalized micro-silica and epoxy resin.
Download figure:
Standard image High-resolution image3.2. FTIR spectra
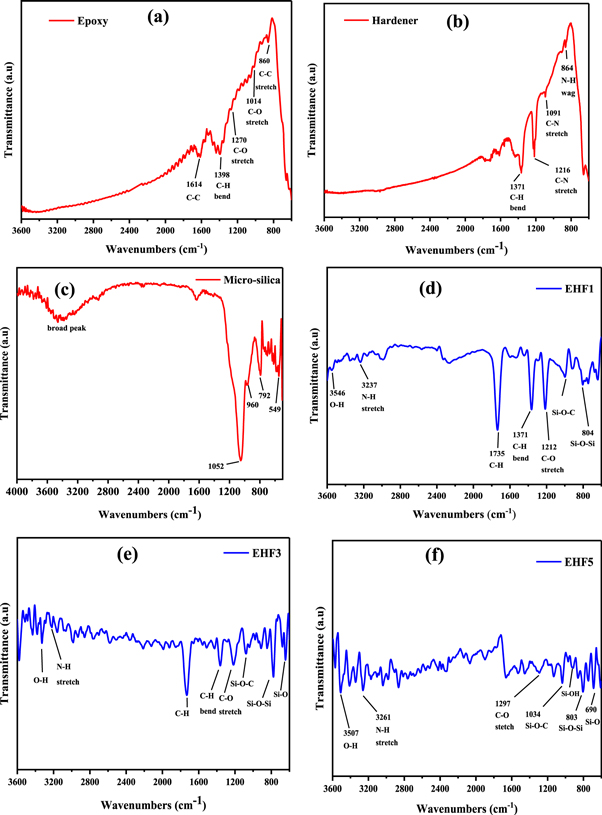
Figures 6(a) and (b) show the FTIR spectra of the pristine epoxy and hardener used. Figure 6(c) presents the FTIR spectra of micro-silica. In the micro-silica spectra, the wavelength range of 3200–3600 cm−1 broadband is related to the stretch vibrations of the OH group. The strong intensity and broad peak at 1052 cm−1 shows Si-O-Si asymmetry. The symmetry peaks of Si-O-Si at 792 cm−1 and 960 cm−1 are stretch vibrations and at 549 cm−1 there is Si-O-Si bend vibration, which describes the characteristic behavior of silica [34]. In figures 6(d), (e) and (f) FTIR spectra demonstrate the ultimate incidence of the chemical reaction between the epoxy resin and amine hardener/functionalized-silica for EHF1, EHF3 and EHF5 respectively. The characteristic intensity peaks are identified and annotated as well. The peaks related to the functional groups of epoxy resin and amine are highlighted at 1300–1000 cm−1 for stretching of C-O of the epoxide group and the N–H stretch peaks occur from 3400–3220 cm−1 respectively. The FTIR spectra of the functionalized micro-filler based epoxy samples show that the silica in the filler, particularly due to silane coating, do the reaction with epoxy, which attribute to asymmetric stretching of Si–O, Si–O–Si, and Si–O–C bonds. Si–O–Si stretch of silica could be obviously detected in the spectra of the resultant epoxy composite coatings [35–37]. The intensity peaks for the C-H aliphatic bending group exist in the range of 1450–1370 and peaks for aliphatic C-H stretching are observed at 2968–2850 cm−1. For primary amines, the N–H bend peaks are in the wavelength range of 1650–1580 cm−1 and for secondary amines, the intensity peaks are in the range of 1580–1500 cm−1. The N–H wag peaks of primary and secondary amines are present from 910–665 cm−1.The absorption band in the range between 1750 and 1625 cm−1 corresponds to the C=O stretch. Also. The C–N stretch for the aliphatic amines is present in the range of 1250–1030 cm−1 and 950-810 cm−1 shows C–C stretching [38–44].
Figure 6. FTIR spectra (a) epoxy (b) hardener (c) micro-silica (d) 1% micro-filler composite coating (e) 3% micro-filler composite coating (f) 5% micro-filler composite coating.
Download figure:
Standard image High-resolution image3.3. XRD spectra analysis
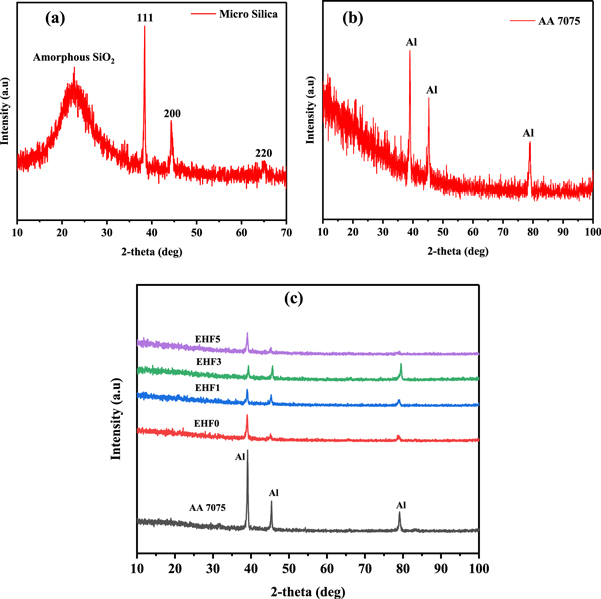
The XRD spectra of micro-silica as received is shown in figure 7(a). The amorphous peak of the silica is at 22.75°. The other peaks are at 38.4°, 44.32°, and 65.07° [45]. The XRD pattern shown in figure 7(b) confirmed the presence of aluminium 2-theta values for the peaks at 38.99°, 45.29°, and 79.05° for the bare aluminium alloy 7075 [46]. The XRD analysis was carried out for comparison of the bare and composite coated samples, presented in figure 7(c). It is obvious from the spectra that the intensity of the peaks was significantly reduced after coating the AA-7075 substrate. This decrease in XRD may possibly be attributed to enhanced absorption of x-rays due to increasing silica content. This behavior can be associated with higher attenuation characteristics of silica also observed in the case of γ-rays previously [47]. The Al peaks are present in the spectra as very high energy x-rays penetrate from the thin coat film and reach the surface of the substrate aluminium alloy 7075.
Figure 7. XRD spectra of (a) micro-silica (b) bare AA 7075 and (c) composite coatings samples.
Download figure:
Standard image High-resolution image3.4. Electrochemical impedance spectroscopy (EIS)
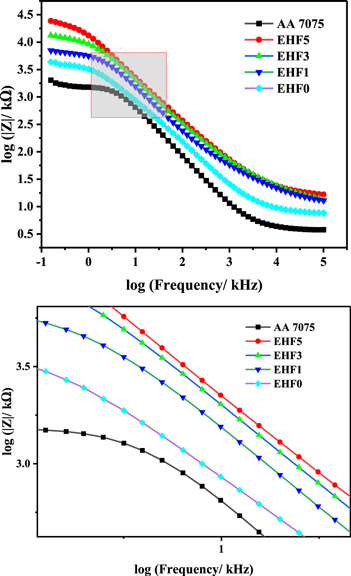
Electrochemical Impedance Spectroscopy (EIS) is the widely used technique to study the actual corrosion resistance and protection efficiency of various types of coating materials applied on different substrates [48, 49]. As EIS is a non-destructive technique as compared to potentio-dynamic characterization, so for long term evaluation of the corrosion samples this method is preferred [50, 51]. Using the EIS technique the effect of micro silica on electrochemical behavior was studied and evaluated the corrosion protection performance of the bare and composite coated samples. Two types of EIS plots were obtained for all samples in 0.6 M NaCl solution. The Bode plot is the graphical representation between the log impedance modulus and log frequency, while the Nyquist plot represents the relationship between real impedance and imaginary impedance. It is obvious from figure 8 that during immersion in the NaCl solution the impedance modulus at the lower value of frequency 0.1 Hz has significantly higher values for the bare as well as epoxy/functionalized SiO2 composite coatings.
Figure 8. Bode plot obtained during the EIS test.
Download figure:
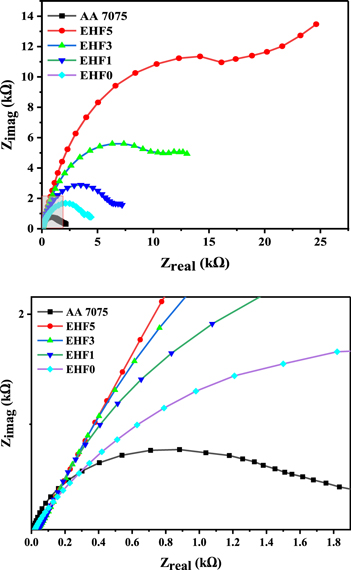
Standard image High-resolution imageSimilarly, figure 9 demonstrates that the composite coating containing 5% functionalized micro-silica has highest corrosion resistance. Moreover, the impedance modulus has higher values for the composite coating containing 5% functionalized micro-silica than the bare and pristine epoxy coatings. These observations show that by dispersing micro-particles in composite coatings, the improvement in the corrosion protection performance occurs for the composite coatings.
Figure 9. Nyquist plot produced during EIS test.
Download figure:
Standard image High-resolution imageIt is used to match the suitable electrochemical equivalent circuit to the experimental EIS data (figure 10). Two equivalent circuits are proposed, one for the bare alloy and the other for coated substrates. Charge transfer resistance (Rct), coating capacitance (Cc), solution resistance (Rs), double layer capacitance (Cdl), coating or pore resistance, and coating capacitance (Cc) are the circuit parameters (Rc).
Figure 10. Electrochemical Equivalent Circuits (EECs) for (a) bare alloy (b) coated samples.
Download figure:
Standard image High-resolution imageRc is a measurement of coating porosity and deterioration and is thought to be caused by the coating's electric resistance to ion transfer through coating pores [52, 53]. Additionally, Rct is a parameter for measuring the electric resistance against electron transfer at the metal/coating interface and is associated with the rate of corrosion [54]. In order to examine the influence of micro filler on the corrosion protection performance of composite coatings, Rc and Rct as well as other parameters were obtained from the EIS data fitting using Echem Analyst software, and are mentioned in table 3. The highest charge transfer resistance (Rct) value was observed for EHF5, which is 22.30 kΩ.
Table 3. EIS parameters measured for bare and coated samples.
| Parameters | |||||
|---|---|---|---|---|---|
| Sample | Cdl (μF) | Rct (kΩ) | Rs(Ω) | Cc(μF) | Rc(kΩ) |
| AA 7075 | 19.27 | 1.583 | 4.117 | -- | -- |
| EHF0 | 14.87 | 3.431 | 8.674 | 7.803 | 38.7 |
| EHF1 | 7.368 | 6.085 | 15.03 | 1.884 | 86.99 |
| EHF3 | 5.853 | 11.41 | 15.99 | 1.813 | 148.9 |
| EHF5 | 5.567 | 22.30 | 19.44 | 1.422 | 215.9 |
Table 3 demonstrates a considerable rise in the Rc and Rct and a decrease in Cdl and Cc values of the epoxy coatings when micro-silica is added to the polymer matrix, indicating composite coatings have a significantly stronger ability to prevent corrosion. Furthermore, it is evident that when 5% micro-silica is added to the epoxy coating, the increase in Rc and Rct is more prominent. The considerable increase in the of Rc value for the EHF5 coated sample could be attributed to the high cross-link density of epoxy due to the development of Si-C bonds inside the epoxy-hardener matrix, which contributes to the improvement in Rc against ionic transfer through the coating pores. Furthermore, substantially greater Rct values for EHF5 samples show that corrosion products and corrosive electrolyte penetration to the coating/metal interface have been constrained. This is due to the high barrier effect of SiO2 particles in the coating matrix as well as an increase in coating adhesion to substrate via forming of the Si-O chemical bonds at the coating/substrate interface.
3.5. Potentiodynamic characterization
The potentiodynamic tests were performed for all the samples to get the Tafel curves in 0.6 M NaCl solution. Figure 11 indicates the polarization curves of bare aluminium alloy 7075 substrate, pure epoxy and hardener coating and epoxy/SiO2 composite coatings containing 1% 3% and 5% of functionalized micro-silica.in 0.6 M NaCl solution. The data fitting was performed for the Tafel curves using Gamry Echem Analyst software to find the values of Ecorr, Icorr and Tafel anodic and cathodic constants βa and βc respectively. Moreover, the corrosion rates (CR) in mm/year were determined using equation (1) [55].
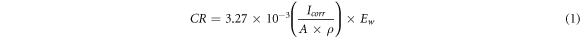
In equation (1), ρ is the density of the aluminium alloy AA 7075 (2.81 g cm−3) and EW is the equivalent weight of the aluminium alloy AA 7075 (9.58 g).
Figure 11. Tafel Curves developed during the potentiodynamic test.
Download figure:
Standard image High-resolution imageThe values of various parameters obtained from the Tafel plot are mentioned in table 4. The findings indicate that composite coatings perform effectively at preventing corrosion since they exhibit much lower Icorr and more positive Ecorr than bare AA 7075 and pure epoxy coatings. Additionally, by dispersing micro-silica filler, the corrosion rate of epoxy coating has greatly decreased. The highest and lowest corrosion rates were recorded for the bare aluminium alloy AA 7075 and EHF5 samples having numerical values of 0.12857 and 0.00287 mm/year respectively. In contrast to bare aluminium alloy AA7075 and epoxy, the barrier performance of epoxy coating is more considerably enhanced by embedding 5% micro-silica because composite coatings containing this amount of SiO2 exhibit a more prominent reduction in corrosion rate and Icorr along with more positive Ecorr values.
Table 4. Potentiodynamic parameters measured for bare and coated samples.
| Samples | Ecorr (mV) | Icorr (μA) | βa (mV/decade) | -βc (mV/decade) | CR (mm/year) |
|---|---|---|---|---|---|
| AA 7075 | −949 | 34.6 | 186.7 | −195 | 0.12857 |
| EHF0 | −886 | 27.51 | 163.3 | −149.6 | 0.10222 |
| EHF1 | −833 | 16.33 | 130.3 | −156.8 | 0.06068 |
| EHF3 | −733 | 2.76 | 105.4 | −187.7 | 0.01030 |
| EHF5 | −643 | 0.772 | 22.9 | −155.4 | 0.00287 |
4. Conclusion
Based on the extracted outcomes in a sequential manner following conclusions can be drawn:
- 1.The charge transfer resistances of EHF0, EHF1, EHF3, and EHF5 are 2.17, 3.84, 7.20, and 14.09 times improved compared to the bare alloy AA 7075. Similarly, the corrosion rate of EHF5 was reduced by 44.79, 35.6, 21.14, and 3.58 folds in comparison to AA-7075, EHF0, EHF1, and EHF3 respectively.
- 2.Functionalized micro-silica successfully inhibited the corrosion of aluminium alloy probably due to enhancement in the cross-linking density of epoxy resin by hindering the diffusion reaction of penetrant ionic species with the matrix material i.e.; epoxy resin.
- 3.Addition of epoxy coating with substrate aluminium alloy 7075 also improved possibly due to strong Si-O bond. As a consequence, barrier properties were also enhanced at the interface of aluminium alloy and composite coatings.
Data availability statement
The data cannot be made publicly available upon publication due to legal restrictions preventing unrestricted public distribution. The data that support the findings of this study are available upon reasonable request from the authors.