Abstract
Quantifying the controls on soil respiration is important for understanding ecosystem physiology and for predicting the response of soil carbon reservoirs to climate change. The majority of soil respiration is typically considered to occur in the top 20–30 cm of soils. In desert soils, where organic matter concentrations tend to be low and plants are deeply rooted, deeper respiration might be expected. However, little is known about the depth distribution of respiration in dryland soils. Here we show that the average depth of soil respiration between pulse precipitation events is almost always greater than 20 cm and is frequently greater than 50 cm in two central New Mexico desert shrublands. The average depth of soil respiration in a piñon-juniper woodland was shallower, between 5 and 40 cm. In the shrublands, 8‰ seasonal variations in the carbon isotope composition of soil-respired CO2 (δ13Cr-soil) that correlate with vapor pressure deficit support root/rhizosphere respiration as the dominant source of soil CO2. Such deep autotrophic respiration indicates that shrubs preferentially allocate photosynthate to deep roots when conditions near the surface are unfavorable. Therefore, respiration rates in these soils are not necessarily correlated with root biomass. The δ13Cr-soil values provide no evidence for CO2 evolved from soil inorganic carbon. Our results also suggest that organic carbon cycling is rapid and efficient in these soils and that the δ13C value of CO2 respired from soils in much of the southwestern US, and perhaps in other semiarid regions, varies seasonally by at least 4‰.
Similar content being viewed by others
Introduction
Carbon dioxide (CO2) emitted from soils to the atmosphere constitutes one of the largest fluxes of carbon to the atmosphere (Raich and Schlesinger 1992). Small but sustained perturbations in the flux of soil-respired carbon could, therefore, drastically alter the CO2 concentration of Earth’s atmosphere. Debate surrounding the sensitivity of soil carbon stocks to global change (for example, Davidson and Janssens 2006) must be resolved to constrain future carbon budgets and predict future climate conditions. Scaling up from individual sites to the global scale will require a mechanistic understanding of soil respiration, which we help to develop by studying the origin of CO2 in central New Mexican soils.
The CO2 flux from dryland (arid and semiarid region) soils, although relatively small on a unit area basis, constitutes a significant portion of the global carbon cycle because drylands cover approximately 40% of Earth’s land surface (Taylor and Lloyd 1992; Shen and others 2008). In dryland soils, uncertainty exists in relative contribution to total soil CO2 efflux from root/rhizosphere respiration (autotrophic respiration), from decomposition of soil organic matter (heterotrophic respiration) and from abiotic sources (for example, calcium carbonate in soils). This uncertainty masks the processes important in the transfer of CO2 from soils to the atmosphere. Investigating the sources of CO2 emitted from dryland soils is therefore important for quantifying the global carbon cycle and, on a smaller scale, for understanding ecosystem carbon exchange in these biomes. In drylands, pulses of biological activity caused by precipitation events punctuate background “between-pulse” levels of biological activity (Noy-Meir 1973). We studied the origin (depth and source) of the “between-pulse” soil-respired CO2 (with the intention to investigate pulse events in the future) to help develop a mechanistic understanding of dryland soil respiration.
Background
Biological CO2 is produced in soils by respiration in the rhizosphere (by plant roots and by associated heterotrophic microorganisms) and by the non-rhizosphere microbial oxidation of organic matter (decomposition). The accumulation of CO2 in soil pore spaces (soil CO2) causes the development of soil-atmosphere concentration gradients, which result in net CO2 diffusion into the atmosphere, a flux typically termed soil-respired CO2. The flux of CO2 from soils is known to be sensitive to soil moisture and temperature, among other variables, but a mechanistic understanding useful for modeling, extrapolation and prediction is lacking.
A mechanistic understanding of soil respiration must involve spatial distribution. For instance, soil respiration rates at discrete depths should ideally be compared with soil temperatures, soil moisture, and so on at those depths. However, the number of studies in which the depth distribution of soil respiration has been investigated is very small compared with the number of studies in which the flux across the soil-atmosphere interface was the only CO2 measurement made. It is typically assumed that soil respiration primarily occurs in the top 20–30 cm of soils and that soil respiration rates below this depth are negligibly small. The concentration of soil organic matter is highest in the near surface soil O and A horizons and decreases exponentially with increasing depth in most soils. Therefore, the assumption that soil respiration is primarily confined to the top several decimeters is probably true for most temperate forest and prairie soils, as suggested by previous studies of soil CO2 profiles (de Jong and Schappert 1972; Dörr and Münnich 1990; Drewitt and others 2005; Hashimoto and others 2007). However, considerable soil respiration below 20 cm soil depth has been documented in some soils (Hirsch and others 2002; Davidson and others 2006) and average depths of soil respiration up to 40 cm have been observed during droughts (Fierer and others 2005; Hashimoto and others 2007).
The depth distribution of roots is also an important consideration for understanding soil respiration, especially in soils with low organic matter contents such as desert soils. Desert shrub roots are known to extend to depths below 5 m (Gile and others 1998) even in soils with petrocalcic horizons (Gibbens and Lenz 2001). These deep roots are known to uptake P (Hartle and others 2006) but their contribution to soil respiration is poorly understood. The average depth of soil respiration varied between 5 and 40 cm in a sand dune with low soil organic matter content planted with a Pinus radiata tree (Cook and others 1998), but soil water was maintained at field capacity in this study and so natural variations were not observed. Naturally occurring depths of soil respiration in desert soils are essentially unknown, despite repeated documentation of such deep rooting systems. The fist objective of this study was to determine the depth of soil respiration in some central New Mexican woodland and shrublands.
A mechanistic understanding of soil respiration must also include an understanding of the relative contribution from different carbon sources to total CO2 efflux. The second objective of this study was to use variations in carbon isotope composition of CO2 produced in soils (δ13Cr-soil) to identify the source of soil-respired CO2. Changes in the value of δ13Cr-soil are controlled by multiple mechanisms, which can be divided into two broad categories.
The first category involves changes in the δ13C value of the substrate supplied to the roots of plants and available for respiration in the rhizosphere. For instance, changes in the magnitude of photosynthetic discrimination (against 13C) are thought to cause changes in the δ13C value of CO2 respired in the rhizosphere. Previous studies (Ekblad and Högberg 2001; McDowell and others 2004a) reported correlations between δ13Cr-soil and vapor pressure deficit (vpd) (saturation vapor pressure—actual vapor pressure). Changes in vpd influence stomatal conductance, which influences the ratio of pCO2 inside leaves to pCO2 in the atmosphere, which in turn influences the discrimination against 13C during photosynthesis and ultimately the isotopic composition of assimilated carbon. Higher relative humidity (that is, low vpd) results in increased stomatal conductance, which causes greater carbon isotope discrimination and hence lower δ13C values of photosynthate (Farquhar and others 1989). The δ13Cr-soil values measured by Ekblad and Högberg (2001) and by McDowell and others (2004a) correlate best with relative humidity or vpd several days prior to collection of CO2 emitted from the soil, suggesting that recently assimilated carbon is rapidly (1–4 days) transported from leaves to the rhizosphere where it can then be respired. In addition to relative humidity, soil moisture might induce a stomatal response that influences δ13Cr-soil (Fessenden and Ehleringer 2003) and δ13Cr (ecosystem-respired CO2) (McDowell and others 2004b) values.
The second category of mechanisms that influence δ13Cr-soil involves changes in the relative contributions of different sources of soil respiration. Steinmann and others (2004) suggested two potential soil processes that might explain the elevated δ13Cr-soil values they observed during the dry, hot summer at their field site near Basel Switzerland. Their first potential explanation for high summer δ13Cr-soil values was an increase in the ratio of rhizosphere to non-rhizosphere respiration. Rhizosphere respired CO2 under seasonally dry conditions should have a higher δ13C value than non-rhizosphere respired CO2 because the source substrate of the former, recent photosynthate, is assimilated with a smaller discrimination than is bulk soil organic matter, most of which was assimilated under more humid conditions. Steinmann and others’ (2004) second explanation, also advocated by Flanagan and others (1999), was an increase in the relative contribution to soil respiration from deeper in the soil, where δ13C values of soil organic matter tend to be higher than they are near the surface (for example, Nadelhoffer and Fry 1988; Melillo and others 1989; Wynn and others 2005; Wynn and others 2006). Laboratory incubation of Alfisols from the Duke Forest Free Air CO2 Enrichment experiment showed that the δ13C value of respired CO2 is 2.2–3.5‰ higher at 4°C than at 22 or 40°C (δ13Cr-soil values at 22 and 40°C were indistinguishable), due to changes in microbial community structure and a resulting change in the substrate being respired (Andrews and others 2000). In deciduous forest plots trenched to remove active roots, Moyes and others (2010) found that the δ13C value of soil-respired CO2 was 5‰ lower during the winter than during the summer. Substantial cooling is therefore thought to increase the δ13C value of heterotrophic CO2 in certain soils and decrease the δ13C value of heterotrophic CO2 in other soils.
In mixed C3–C4 ecosystems, variations in the relative proportion of C3 versus C4 rhizosphere respiration might cause large variations in δ13Cr-soil values. The efficiency of C4 photosynthesis is known to increase in comparison with C3 photosynthesis as temperature increases (Ehleringer 1978; Ehleringer and others 1997) and temperature is therefore thought to control the seasonal transition from C3 to C4 dominated photosynthesis in prairie ecosystems (Kemp and Williams 1980; Ode and others 1980; Lai and others 2006). If photosynthesis drives rhizosphere respiration (Högberg and others 2001) then changes in the relative proportion of C3 versus C4 photosynthesis should result in changes in the relative proportion of C3 versus C4 rhizosphere respiration. Lai and others (2006) attributed the seasonal increase in the δ13C value of total ecosystem respiration in a tallgrass prairie to a relative increase in the contribution from C4 grasses, which in turn was explained by the decreasing light use efficiency of C3 plants with increasing temperature.
To help develop a mechanistic understanding of dryland soil respiration we tested two hypotheses concerning semiarid and arid regions where O horizons are absent, soil A horizons contain little organic matter and plants are deeply rooted: (1) respiration occurs throughout the top meter of soil rather than being confined to the top several decimeters, (2) in these desert soils with low organic matter contents, autotrophic respiration dominates the soil-atmosphere CO2 flux. We tested hypothesis 1 by measuring profiles of soil CO2 concentration in shrubland and woodland ecosystems in semi arid central New Mexico. We tested hypothesis 2 by measuring seasonal changes in the carbon isotope composition of soil-respired CO2.
Methods
Study Sites
The study was conducted on the Sevilleta National Wildlife Refuge (SNWR) in central New Mexico (Figure 1). New Mexico has a semiarid monsoon climate. Mean annual precipitation varies strongly with elevation. In low elevation shrublands and grasslands approximately 70% of annual precipitation occurs during July, August, and September, whereas in conifer woodlands approximately 50% of annual precipitation occurs during these three summer months (Anderson-Teixeira and others 2011). We collected soil CO2 from three biomes that characterize much of New Mexico and the semiarid southwestern United States (1) Piñon-juniper woodland (PJ), (2) Great Basin shrubland (GBS), and (3) Chihuhuan Desert shrubland (CDS). One site in each of these biomes was chosen for this study (Figure 1); the same sites were used in Breecker and others (2009). The Chihuhuan Desert shrubland that we studied is nearly monospecific, dominated by the C3 shrub creosote bush (Larrea tridentata). All the sites were located on recently abandoned sandy channel alluvium. The soils at each site are probably late Holocene in age and have undergone only very weak pedogenesis. The soil at the piñon-juniper site has a 20 cm thick ochric (light-colored) A horizon but A horizons in the other soils are thin and very poorly developed. Weak Ck (minimal pedogenic alteration with the exception of soil-formed calcite coatings on the underside of clasts) horizons underlie the ochric A horizons in all the soils. There are no petrocalcic horizons in these soils unlike other areas on the SNWR. Vegetation at all four sites is a mixture of C3 and C4 plant species.
Study location map. The biomes studied are representative of much of the southwestern United States of America. The Great Basin shrubland (studied here) is similar, but not identical to the Colordao Plateau Shrub Steppe biome pictured on the map. PJ Piñon-Juniper Woodland; CDS Chihuahuan Desert Shrubland; GBS Great Basin Shrubland.
Soil CO2 Collection and Analysis
The sample collection and analytical methods are described in detail by Breecker and Sharp (2008). Nine or ten septum-capped soil gas wells constructed of 1 m lengths of ¼” (6.35 mm) O.D. stainless steel tubing, crimped shut at one end, were installed horizontally in the top meter of the soil at each site. Slits were created in the sidewall of the wells to allow gas entry. The volume of the soil gas wells was approximately 16 ml. Soil gas samples (0.2–1.5 ml) were collected from the wells using a syringe approximately once per month beginning in September 2006 and continuing through May 2008. Most of the soil gas samples were collected at least 5 days after the last measurable precipitation event. With only a few exceptions (dates specified below), when precipitation did occur less than 5 days before collection, less than 1 cm of precipitation was recorded over the 5 days prior to soil gas collection. The soil gas samples measured in this study are representative of the pore space CO2 at the slits in the wells (a sub cm depth resolution). The natural CO2 gradient was not disturbed by monthly gas collection because the syringe samples consisted entirely of gas from the well itself, which had equilibrated with gas in the pore space immediately surrounding the well opening. The concentration and stable isotope composition of CO2 in the gas samples was determined in the stable isotope laboratory at the University of New Mexico using the automated technique described in Breecker and Sharp (2008). Carbon isotope compositions are reported here in the standard delta notation relative to VPDB calibrated to a δ13C value of NBS-19 = 2.02‰ (Hut 1987). During each sampling campaign, samples of atmospheric air were collected and soil temperature was measured at multiple depths using buried TC-PVC Omega® thermocouples and a handheld meter. The soil CO2 and temperature data used in this study were reported in a previous study (Breecker and others 2009). Air temperature and vapor pressure data (available from the Sevilleta Long Term Ecological Research project website: http://sev.lternet.edu) averaged over the daylight hours 2 weeks prior to each sampling campaign were used to calculate vpd.
Soil CO2 Models
Average depths of soil respiration (z avg) and soil respiration rates were determined by fitting CO2 profiles calculated using a numerical production-diffusion model to measured soil CO2 profiles. A least squares minimization program was used to find best-fit values for z avg. Fick’s second law of diffusion can be expressed for soil CO2 as:
where C is the CO2 concentration in soil pores, t is time, D s is the effective diffusion coefficient for CO2 in the soil, z is depth and P(z) is the depth dependent production rate of CO2. We solved equation (1) using a finite difference predictor–corrector method. We used the following equation to calculate D s (Bird and others 1960; Moldrup and others 2000):
where D air is the diffusion coefficient for CO2 in air, θa is the free air porosity, θt is the total porosity, P 0 is the air pressure at sea level, P is the air pressure at the elevation of interest, T 0 is 25°C, and T is the soil temperature in °C. We used measured soil temperatures at 40–60 cm for T (reported in Breecker and others 2009) and the elevation of each site to calculate P. The average depth of respiration and vertical changes in free air porosity both control the curvature of soil CO2 concentration profiles. Deeper respiration and decreases in free air porosity with depth both result in less profile curvature when compared with shallower respiration and constant porosity with depth. We estimated the shallowest average depths of soil respiration (and the corresponding maximum soil respiration rates) that are consistent with our measured soil CO2 concentrations by considering a maximum vertical decrease in free air porosity. We assumed constant total porosity with depth, which is justified due to minimal pedogenesis (no B horizons) and the consistent texture (all sand) of these soils. We also assumed the maximum increase in water content with depth in the soils studied is equivalent to the maximum increase in water content with depth measured using time domain reflectometry at another site on the SNWR (Deep Well) where soil moisture has been monitored from the time period 1996–1999. We subtracted these measured volumetric water contents from 0.5, a typical value for the total porosity of unconsolidated sand (Pryor 1973) to get free air porosity as a function of depth. We used an exponential fit to the calculated free air porosities (Figure 2) as input into our CO2 production–diffusion model.
Profiles of soil water content and soil porosity. The water content profile shows the largest observed vertical change in water content at Deep Well, another SNWR site where soil moisture was measured continuously from 1996–1999 (Sevilleta LTER). These water contents were subtracted from a total porosity of 0.5 to estimate the maximum vertical change in free air porosity for the soils studied here. The equation for free air porosity that is shown was used to calculate the diffusion coefficient for CO2 in the soils.
We compared the effects of using two different functions for the production term in equation (1): an exponential function and a step function. The exponential function used was (Hesterberg and Siegenthaler 1991; Cerling 1999):
where z 0 is the characteristic depth: z 0 = z avg/0.693, and the step function was:
where z top and z bot refer to the shallowest and deepest depths, respectively, at which soil respiration occurs z avg = (z top − z bot)/2 and R is a constant. The comparison was intended to test the effect of different depth distributions of respiration on calculated values of z avg.
The δ13C value of soil-respired CO2 was calculated from measured soil CO2 and atmospheric CO2 values using the following equation rearranged from Davidson (1995):
where δ is the standard delta notation for carbon isotope compositions, C is concentration and the subscripts J, a, and s refer to CO2 respired in the soil, CO2 in the atmosphere and soil pore space CO2, respectively. The technique of calculating δ13Cr-soil values using below-ground measurements yields the isotopic composition of CO2 produced in the soil while collection of soil gas in above-ground chambers (for example, Flanagan and others 1999) yields the isotopic composition of CO2 diffusing into the atmosphere. At steady state, these fluxes are identical. Under transient conditions, however, CO2 diffusing across the soil–atmosphere interface does not typically have the same δ13C value as CO2 produced in the soil. The concentration and isotopic composition of soil CO2 at depth change slowly and are therefore typically at steady state whereas profiles are more often transient near the surface (because soil temperature and soil moisture change more rapidly there). Therefore, chamber-based measurements provide high temporal resolution, but are likely to reflect a transient CO2 flux (Nickerson and Risk 2009; Moyes and others 2010). Equation (2) assumes steady state conditions, so we used soil CO2 collected from the four deepest wells at each site (50–100 cm) to calculate δ13Cr-soil. Deep soil CO2 responds more slowly than does shallow CO2 to changes that occur near the surface so our technique is appropriate for investigating seasonal variation but not daily variation. In addition, deep soil CO2 is probably minimally influenced by the decomposition of litter at the soil surface.
Vegetation Surveys and Organic Matter Analysis
Vegetation surveys were conducted on July 26, 2007 to estimate the proportions of different plant species at each site. The number of individuals and cover class (an estimate of the percentage cover based on the following bins: <1/32, 1/32–1/16, 1/16–1/8, 1/8–1/4, 1/4–1/2, 1/2–3/4, >3/4) in a 1 × 1 m square were determined for each species every 2 m along transects through a previously determined around 500 m2 area appropriate for each site. The carbon isotope composition of dried and powdered stem/leaf mixtures of each plant species were measured using a Costech Elemental Analyzer coupled with a Delta Plus Mass Spectrometer in the stable isotope laboratory at the University of New Mexico. The carbon isotope composition of soil organic matter was measured using the same technique. Living roots were removed from the soil samples which were subsequently treated with 10% HCl to remove calcium carbonate before analysis. A pure graphite standard was used to calibrate peak area as a measure of the weight fraction of organic carbon in soil samples (estimated error equals ±0.25 wt%). The concentration of organic carbon was then calculated based on the mass of soil analyzed and measured peak area.
Results
Winter CO2 profiles were characterized by low measured CO2 concentrations (<~1,000 and <~2,500 ppm V at the shrubland and woodland sites, respectively), little curvature and δ13C values that decrease gradually with depth, reaching the lightest measured values at depths below 50 cm (Figure 3). In contrast, profiles during warm, wet periods (summer and early autumn) were characterized by higher CO2 concentrations (up to ~3,000 and ~8,000 ppm V at the shrubland and woodland sites, respectively) and more curvature than winter profiles and δ13C values that decrease abruptly with depth in the shallow subsurface but change little with depth below 20–30 cm (Figure 3). Profiles during dry, warm periods (late spring, summer) were similar to winter profiles except that CO2 concentration profiles have less curvature and the δ13C values during dry periods were higher at all depths than during the winter (Figure 3). All measured CO2 profiles were reported by Breecker and others (2009).
The soil at the piñon-juniper site has higher organic matter contents than the other soils, consistent with the observation of a darker and thicker (20 vs. <5 cm) ochric A horizon in this soil (Table 1). The soils at the Great Basin shrubland sites have organic matter contents below 0.1 wt% (Table 1). In general, the ecosystems studied consist of C4 grasses, which have the highest δ13C values, C3 shrubs or trees, which have intermediate δ13C values, and C3 forbs, which have the lowest δ13C values (Table 2). The Great Basin shrubland site has C4 shrubs with δ13C values higher than the δ13C values of grasses growing at the site (Table 2).
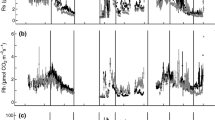
Compared with annual median values, the average depth of soil respiration in all soils studied was shallower following precipitation events, deeper during the coldest time of the year and deepest during the driest times of the year (Figure 4). Average depths of respiration in the shrublands during the first half of the study period (Septemeber 2006–June 2007) were shallower than during the second half of the study period (June 2007–May 2008), when average depths of respiration in the shrubland soils were typically below 50 cm (Figure 4). CO2 measurements made in the top meter cannot be used to quantify average depths below about 60 cm assuming exponentially decreasing CO2 production and free air porosity because there is not enough curvature in the top meter of these soil CO2 profiles. Therefore, where the average depth of soil respiration is about 60 cm as shown in Figure 4, it is possible that the actual average depth of soil respiration was much greater. Furthermore, if the step function CO2 production equation is used instead of the exponentially decreasing production, calculated minimum average depths are deeper by 1.3–3 times; the average depths during the dry periods of 2007–2008 and are greater than 80 cm. If a constant porosity of 0.4 is used, the best-fit average depths during the dry periods of 2007–2008 are greater than 1 m.
Time series of δ13Cr-soil, zavg, soil respiration rate, soil temperature, and precipitation. δ13Cr-soil, zavg, and soil respiration rate were calculated using measured concentrations and δ13C values of pore space soil CO2 as discussed in the methods section of the text. Estimates of minimum (shallowest) values for z avg and maximum values for respiration rate are shown. Values for z avg were calculated assuming an exponential decrease in soil respiration rates with depth because this resulted in shallower values than the step function described in the text. Soil temperatures are shown for ~20 cm and for the depth of measurement closest to the deepest calculated values of z avg for each soil. Note change in scale between woodland and shurbland plots of soil respiration rate. Open symbols indicate samples likely influenced by pulse precipitation (>1 cm of precipitation was recorded during the 5 days prior to sampling collection). Precipitation was measured at meteorological stations maintained by D. Moore in association with the Sevilleta Long Term Ecological Research Project. No precipitation data are reported for the Chihuahuan Desert Shrubland site because there are no meteorological stations in sufficient proximity.
δ13Cr values calculated by constructing soil CO2-based keeling plots and subtracting 4.4‰ from the y-intercept are generally within ±0.2‰ of the values show in Figure 4. Estimated uncertainties (using Monte Carlo Simulations) of calculated δ13Cr-soil values are approximately ±0.2‰ for samples in which CO2 concentrations are approximately 2,000 ppm V or greater and increase as CO2 concentrations decrease such that the uncertainties of winter and dry spring/summer δ13Cr-soil values are approximately ±1.0‰. The δ13Cr-soil values calculated from the soil CO2 data varied seasonally by 7–8‰ at the shrubland sites and by around 4‰ at the woodland site. δ13Cr-soil values were highest in the summer and lowest in the winter. The values of δ13Cr-soil correlated well with both soil temperature (at 20 cm) and vpd (Figure 5).
Discussion
The results of this study indicate that large variations in the average depth of respiration and the carbon isotope composition of soil-respired CO2 occur in all of the soils studied. The seasonal and interannual variations in the average depth and the δ13C value of soil-respired CO2 are especially large in the shrubland soils. In the shrubland soils CO2 concentration profiles during dry periods have little curvature in the top 1 meter, indicating deep respiration. Taken together, and as discussed below, the large variations in zavg and δ13Cr-soil in the shrublands suggest that large changes occur in the relative activity of shrub roots at different soil depths.
Soil CO2 and Soil-Respired CO2
Large seasonal changes in δ13Cr-soil values (as high as 8‰) were observed at each site (Figure 4), but the measured δ13C value of soil CO2 only varied by about 2‰ at each site (Figure 3). This occurs because low winter respiration rates nearly balance the effect of decreasing δ13Cr values during the fall and winter. Low respiration rates cause a decrease in the amount of biogenic CO2 in the soil and a concomitant increase in the relative proportion of atmospheric CO2, which has a less negative δ13C value than biogenic CO2. Whereas respiration rates also decreased during dry episodes in the spring and summer during the period of study, the decrease was only sufficient to generate a significant effect from atmospheric mixing in March and May of 2008. Continued decrease of respiration rates could result in very high δ13C values of soil CO2 during the dry period of the spring and summer. These observations and theoretical considerations indicate that the δ13C value of soil CO2 in these soils is controlled by both the rate of respiration and the value of δ13Cr-soil although it is possible that other factors such as carbon isotope fractionation associated with transient state diffusion (for example, Brüggemann and others 2011) influence the δ13C values of CO2 in these soils. The cause of seasonal variation in δ13Cr values is discussed below.
The Source of Between-Pulse Soil-Respired CO2
We argue that the large seasonal variation of δ13Cr-soil values observed in this study result from changes in the magnitude of photosynthetic discrimination that are expressed in rhizosphere respiration and that between-pulse soil-respired CO2 is almost entirely autotrophic. Before we discuss the evidence for this explanation, we first eliminate other potential explanations for seasonal variations in the values of δ13Cr-soil: (1) a change from C3- to C4-dominated rhizosphere respiration, (2) variable mixing ratios of biogenic CO2 with CO2 from a geologic source (that is, magmatic CO2 or CO2 from dissolution/precipitation of calcite), (3) a change in the vertical distribution of decomposition rates, (4) changes in the δ13C value of soil organic matter, (5) changes in the relative decomposition rates of different organic substrates (substrate shift), and (6) variable proportions of rhizosphere respiration and decomposition.
Mechanism 1 (change in C3/C4 respiration) is perhaps the most obvious potential explanation for seasonally variable δ13Cr-soil values in mixed C3–C4 communities. However, mechanism 1 does not provide a satisfying explanation because there is no correlation between the fraction of C4 vegetation and the magnitude of the observed changes in values of δ13Cr-soil. For instance, a similar seasonal shift in δ13Cr-soil values is observed at the Chihuahuan Desert site, where C4 plants constitute a very small fraction of vegetative cover (the site is nearly monospecific, dominated by the C3 shrub L. tridentata), and the Great Basin shrubland site, where C4 plants constitute approximately 50% of the vegetative cover. Whereas mean annual δ13Cr-soil values do correlate with the relative abundance of C3 and C4 vegetation (mean δ13Cr-soil values are higher at the Great Basin shrubland site than at the Chihuahuan Desert site), the seasonal variation of δ13Cr-soil values measured in this study must be explained by some other process. It remains possible that seasonal changes in C3–C4 productivity drive variations in δ13C value of CO2 respired in the shallow subsurface of these soils (<20 cm, where the root density of C4 grasses is the highest) but such variations would require higher temporal resolution data to resolve.
The biannual (winter and dry periods during the summer) decrease in soil CO2 concentrations allows the potential for a geologic source of CO2 (mechanism 2) to be evaluated. We need to consider magmatic CO2 as a possible source in the soils we studied given the evidence for a deep CO2 source and the location of our study area in proximity to a magma chamber beneath Socorro, New Mexico (Sandford and others 1977). A small, but presumably constant contribution from a deep magmatic source with a relatively high δ13C value (~−6‰, compared to biological sources which have δ13C values <−12‰) would be discernible as the biologic flux diminishes. The large range in δ13Cr-soil values when soil CO2 concentrations are low indicate that a negligible component of the between-pulse CO2 produced at depth in these soils originates from a magmatic source. Inorganic carbon in these soils is another potential abiotic source of CO2. Calcite in these soils has a δ13C value of around 0‰. The low δ13Cr-soil values measured in this study are consistent with entirely biological CO2 sources and indicate that the contribution of CO2 evolved from calcite is minimal, even though the abiotic contribution cannot be quantified. The dominance of the biological CO2 flux over the carbonate weathering CO2 flux, even in dryland soils containing abundant calcium carbonate, is also supported by results from Serna-Pérez and others (2006). Pulse precipitation may result in the production of CO2 from the solution and eventual precipitation of calcite in soils but it is currently unclear whether or not this flux constitutes a significant component of total soil respiration after precipitation events.
Low organic carbon contents occur in these soils because they experience high temperatures on a seasonal basis and contain high concentrations of oxidative enzymes (Stursova and Sinsabaugh 2008). Rapid oxidation of organic matter indicates that decomposition rates are probably limited by litter input rates, especially at depth. High decomposition rates could potentially be supported near the surface in these soils but decomposition is probably very minor at depth where litter input is small. Combining the observation that the average depth of respiration in the shrubland soils is almost always greater than 20 cm and frequently greater than 50 cm with the argument that decomposition is probably minimal at depth suggests that the contribution of decomposition to the measured soil respiration is minor. Therefore, mechanisms 3–6 are not the most realistic explanations for changes in δ13Cr-soil.
There is additional evidence against mechanisms 3–6. The lack of a correlation between the average depth of respiration and values of δ13Cr-soil in the woodland soil studied here (Figure 6) suggests that a change in the vertical distribution of decomposition (mechanism 3) is not the primary cause of the observed variation in δ13Cr-soil. The observation that the average depth of respiration is below 50 cm during dry periods when δ13Cr-soil values are least negative and during cold periods when δ13Cr-soil values are most negative precludes mechanism 4 because it is highly unlikely that the δ13C value of soil organic matter at greater than 50 cm depth changes by 8‰ on a seasonal basis. In the shrublands, the δ13Cr-soil values during the winters of 2006–2007 and 2007–2008 were similar (minimum values occurred in January and were −26.7 and −26.9 for GBS and −29.5 and −29.2 for CDS in 2007 and 2008, respectively) but the average depths of respiration were deeper, and the soil temperatures at the average depths of respiration were more than 6°C warmer during the winter of 2007–2008 than the previous winter (Figure 4). Therefore, a soil temperature control on the heterotrophic microbial community and the substrate decomposed by it (mechanism 5) is not the best explanation for variations in δ13Cr-soil values. Furthermore, a temperature-driven heterotrophic control on δ13Cr-soil would require a sensitivity that is at least two times higher in the shrublands than in the woodland soil because seasonal soil temperature variations in the woodland and shrubland soils are similar but seasonal variations in δ13Cr-soil values are two times larger in the shrubland soils. A substrate shift from labile, relatively less 13C-depleted root exudates to recalcitrant, more 13C-depleted (Park and Epstein 1961; Benner and others 1987) organic compounds as photosynthesis, and thus the delivery of labile photosynthate to the rhizosphere, decreases cannot explain the observed variation in δ13Cr-soil. Although δ13Cr-soil values are relatively low when the rate of photosynthesis is low in the winter, δ13Cr-soil values are at the high end of the observed range when the rate of photosynthesis is low during the dry part of the late spring/early summer.
The δ13C value of organic matter in the soils studied is approximately equal to or slightly higher than the highest δ13Cr-soil values measured. Therefore, an increase in the relative contribution of decomposition to total soil respiration (mechanism 6) with increasing temperature could explain the approach of δ13Cr-soil toward the δ13C value of soil organic matter. However, when compared with decomposition, rhizosphere respiration is thought to be more sensitive to temperature (Boone and others 1998), in which case δ13Cr-soil values should approach the δ13C value of soil organic matter as temperature decreases, not as temperature increases.
We argue that seasonal changes in the magnitude of photosynthetic discrimination during C3 photosynthesis are primarily responsible for the observed variability in δ13Cr-soil. This interpretation is strongly supported by the correlation between vpd and δ13Cr-soil (Figure 5). We argue that the correlation between 20 cm soil temperature and δ13Cr-soil is not causal, but instead occurs because shallow soil temperature correlates with air temperature (Figure 5) and air temperature largely controls vpd. The argument for a stomatal control of δ13Cr-soil is further supported by the similar relationship between vpd and the δ13C value of ecosystem respiration that has been observed in conifer forests of western Oregon, where C4 plants are absent (Bowling and others 2002) (Figure 7).
Comparison of the relationship between δ13Cr and vapor pressure deficit (vpd) observed in different ecosystems. δ13Cr values from Bowling and others (2002) are for total ecosystem respiration and were interpreted to reflect changes in the magnitude of photosynthetic discrimination. The correlation with vpd and the similarity of the soil-respired CO2 curves from this study with the ecosystem-respired CO2 curves from Bowling and others (2002) support the conclusion that the magnitude of photosynthetic discrimination controls δ13Cr-soil in the soils studied.
The arguments above suggest that the between-pulse CO2 in soils studied here is primarily produced by rhizosphere respiration. Without knowing the total range of pure autotrophic δ13Cr values for each soil, we cannot quantitatively partition total soil respiration into its autotrophic and heterotrophic components using the carbon isotope data presented here. However, we suggest that the observed 8‰ range is almost as large as the total autotrophic range in δ13Cr values and therefore that autotrophic respiration dominates in these soils. The smaller range of δ13Cr-soil values at the piñon-juniper site in comparison to the other sites likely results from decomposition attenuating the seasonal change in δ13Cr-soil values caused by rhizosphere respiration. The higher organic matter content in the soil at the piñon-juniper site supports this interpretation. CO2 from 50 to 100 cm depth in the shrubland soils, however, likely provides a unique opportunity to study rhizosphere respiration minimally diluted by SOM decomposition.
Heterotrophic respiration in the shallow subsurface is thought to respond quickly to pulse precipitation and to dominate the soil-to-atmosphere CO2 flux during small precipitation events (Austin and others 2004; Schwinning and Sala 2004). The origin of CO2 respired during pulse events is more difficult to characterize using stable carbon isotope ratios than is the origin of between-pulse CO2 due to isotope fractionation associated with the transient nature of pulse events (Nickerson and Risk 2009; Moyes and others 2010). These difficulties should be addressed at some point. However, the majority of annually integrated total ecosystem respiration occurs between, not during, pulse events; in shrubland ecosystems in central New Mexico approximately 85% of total annual ecosystem respiration occurs between pulse events (Litvak unpublished data). Therefore, the results of the present study suggest that the majority of CO2 respired annually by these desert shrubland ecosystems is autotrophic in origin and is sourced directly from recent photosynthate. Combined with the low organic matter contents in these soils, dominance of autotrophic respiration indicates carbon is rapidly and efficiently cycled through these shrubland ecosystems; a substantial portion of the carbon must have a mean residence time that approaches the transport time of photosynthate from leaves to roots.
Conclusions
Measurements of the depth of soil respiration and the carbon isotope composition of respired CO2 can be used to study soil respiration and its sources. The observed large variations in the depth of respiration and δ13Cr-soil values are best explained by root/rhizosphere respiration expressing changes in the magnitude of photosynthetic discrimination during C3 photosynthesis. We therefore conclude that the between-pulse flux of CO2 from these soils is largely autotrophic and not from decomposition of soil organic matter, weathering of calcium carbonate or from a magmatic source. Smaller variations in the depth of respiration and δ13Cr-soil values at the piñon-juniper site can be explained by dilution of autotrophic CO2 by decomposition in the A horizon of that soil. If CO2 respired in the shrubland soils is primarily autotrophic in origin, then the relative activity of shrub roots at different depths must change seasonally and interannually, perhaps due to preferential allocation of photosynthate to roots at different soil depths, and the shrubland soils should be considered as excellent natural laboratories for the study of rhizosphere respiration. We also conclude that carbon is rapidly and efficiently cycled through these ecosystems (especially the shrublands). Given that the biomes we studied are representative of regional vegetation and that seasonal variations in vapor pressure deficit are largely temperature driven and therefore regionally consistent, it is likely that δ13C values of CO2 respired from soils across much of the southwestern United States change seasonally by at least 4‰.
References
Anderson-Teixeira KJ, DeLong JP, Fox AM, Brese DA, Litvak ME. 2011. Differential responses of production and respiration to temperature and moisture drive the carbon balance across a climatic gradient in New Mexico. Global Chang Biol 17:410–24.
Andrews JA, Matamala R, Westover KM, Schlesinger WH. 2000. Temperature effects on the diversity of soil heterotrophs and the δ13C of soil-respired CO2. Soil Biol Biochem 32:699–706.
Austin AT, Yahjian L, Stark JM, Belnap J, Porporato A, Norton U, Ravetta DA, Schaeffer SM. 2004. Water pulses and biogeochemical cycles in arid and semiarid ecosystem. Oecologia 141:221–35.
Benner R, Fogel ML, Sprague EK, Hodson RE. 1987. Depletion of 13C in lignin and its implications for stable carbon isotope studies. Nature 329:708–10.
Bird RB, Stewart WE, Lightfoot EN. 1960. Transport phenomena. New York: Wiley.
Boone RD, Nadelhoffer KJ, Canary JD, Kaye JP. 1998. Roots exert a strong influence of the temperature sensitivity of soil respiration. Nature 396:570–2.
Bowling DR, McDowell NG, Bond BJ, Law BE, Ehleringer JR. 2002. 13C content of ecosystem respiration is linked to precipitation and vapor pressure deficit. Oecologia 131:113–24.
Breecker D, Sharp ZD. 2008. A field and laboratory method for monitoring the concentration and stable isotope composition of soil CO2. Rapid Commun Mass Spectrom 22:449–54.
Breecker D, Sharp ZD, McFadden L. 2009. Seasonal bias in the formation and stable isotope composition of pedogenic carbonate in modern soils from central New Mexico, USA. Bull Geol Soc Am 121:630–40.
Brüggemann N, Gessler A, Kayler Z, Keel SG, Badeck F, Barthel M, Boeckx P, Buchmann N, Brugnoli E, Esperschütz J, Gavrichkova O, Ghashghaie J, Gomez-Casanovas N, Keitel C, Knohl A, Kuptz D, Palacio S, Salmon Y, Uchida Y, Bahn M. 2011. Carbon allocation and carbon isotope fluxes in the plant-soil-atmosphere continuum: a review. Biogeosciences Discussions 8:3619–95.
Cerling TE. 1999. Stable carbon isotopes in palaeosol carbonates. In: Thiry M, Simon-Coincon R, Eds. Palaeoweathering, palaeosurfaces and related continental deposits. Oxford: Blackwell, Special Publication of the International Association of Sedimentologists, pp 43–60.
Cook FJ, Thomas SM, Kelliher FM. 1998. A model of one-dimensional steady-state carbon dioxide diffusion from soil. Ecol Model 109:155–64.
Davidson EA, Janssens IA. 2006. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–73.
Davidson EA, Savage KE, Trumbore SE, Borken W. 2006. Vertical partitioning of CO2 production within a temperature forest soil. Global Chang Biol 12:944–56.
Davidson GR. 1995. The stable isotope composition and measurement of carbon in soil CO2. Geochim Cosmochim Acta 59:2485–9.
de Jong E, Schappert HJV. 1972. Calculation of soil respiration and activity from CO2 profiles in the soil. Soil Sci 113:328–33.
Dörr H, Münnich KO. 1990. 222Rn flux and soil air concentration profiles in West-Germany. Soil 222Rn as a tracer for gas transport in the unsaturated soil zone. Tellus 42B:20–8.
Drewitt GB, Black TA, Jassal RS. 2005. Using measurements of soil CO2 efflux and concentrations to infer the depth distribution of CO2 production in a forest soil. Can J Soil Sci 85:213–21.
Ehleringer JR. 1978. Implications of quantum yield differences to the distributions of C3 and C4 grasses. Oecologia 31:255–67.
Ehleringer JR, Cerling TE, Helliker BR. 1997. C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112:285–99.
Ekblad A, Högberg P. 2001. Natural abundance of 13C in CO2 respired from forest soils reveals speed of link between tree photosynthesis and root respiration. Oecologia 127:305–8.
Farquhar GD, Ehleringer JR, Hubick KT. 1989. Carbon isotope discrimination and photosynthesis. Ann Rev Plant Physiol Plant Mol Biol 40:503–37.
Fessenden JE, Ehleringer JR. 2003. Temporal variation in d13C of ecosystem respiration in the Pacific Northwest: links to moisture stress. Oecologia 136:129–36.
Fierer N, Chadwick OA, Trumbore SE. 2005. Production of CO2 in soil profiles of a California annual grassland. Ecosystems 8:412–29.
Flanagan LB, Kubien DS, Ehleringer JR. 1999. Spatial and temporal variation in the carbon and oxygen stable isotope ratio of respired CO2 in a boreal forest ecosystem. Tellus 51B:367–84.
Gibbens RP, Lenz JM. 2001. Root systems of some Chihuahuan Desert plants. J Arid Environ 49:221–63.
Gile LH, Gibbens RP, Lenz JM. 1998. Soil-induced variability in root systems of creosotebush (Larrea tridentata) and tarbush (Flourensia cernua). J Arid Environ 39:57–78.
Hartle RT, Fernandez GCJ, Nowak RS. 2006. Horizontal and vertical zones of influence fro root systems of four Mojave Desert shrubs. J Arid Environ 64:586–603.
Hashimoto S, Tanaka N, Kume T, Yoshifuji N, Hotta N, Tanaka K, Suzuki M. 2007. Seasonality of vertically partitioned soil CO2 production in temperature and tropical forest. J For Res 12:209–21.
Hesterberg R, Siegenthaler U. 1991. Production and stable isotopic composition of CO2 in a soil near Bern, Switzerland. Tellus Series B Chem Phys Meteorol 43:197.
Hirsch AI, Trumbore SE, Goulden M. 2002. Direct measurements of the deep soil respiration accompanying seasonal thawing of a boreal forest soil. J Geophys Res 108:8221.
Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Högberg MN, Nyberg G, Ottosson-Löfvenius M, Read DJ. 2001. Large scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–92.
Hut G. 1987. Consultant’s group meeting on stable isotope reference samples for geochemical and hydrological investigations. Vienna: International Atomic Energy Agency.
Kemp PR, Williams GJ. 1980. A physiological basis for niche separation between Agropyron smythii (C3) and Bouteloua gracilis (C4). Ecology 61:846–58.
Lai C-T, Riley W, Owensby C, Ham J, Schauer A, Ehleringer JR. 2006. Seasonal and interannual variations of carbon and oxygen isotopes of respired CO2 in a tallgrass prairie: measurements and modeling results from 3 years with contrasting water availability. J Geophys Res 111:D08S06.
McDowell NG, Bowling DR, Bond BJ, Irvine J, Law BE, Anthoni P, Ehleringer JR. 2004a. Response of the carbon isotopic content of ecosystem, leaf, and soil respiration to meteorological and physiological driving factors in a Pinus ponderosa ecosystem. Global Biogeochem Cycles 18:GB1013.
McDowell NG, Bowling DR, Schauer A, Irvine J, Bond BJ, Law BE, Ehleringer JR. 2004b. Associations between carbon isotope ratios of ecosystem respiration, water availability and canopy conductance. Global Chang Biol 10:1767–84.
Melillo JM, Aber JD, Linkins AE, Ricca A, Fry B, Nadelhoffer KJ. 1989. Carbon and nitrogen dynamics along the decay continuum: plant litter to soil organic matter. Plant Soil 115:189–98.
Moldrup P, Olesen T, Gamst J, Schjonning P, Yamaguchi T, Rolston DE. 2000. Predicting the gas diffusion coefficient in repacked soil: water-induced linear reduction model. Soil Sci Soc Am J 64:1588–94.
Moyes AB, Gaines SJ, Siegwolf RTW, Bowling DR. 2010. Diffusive fractionation complicates isotopic partitioning of autotrophic and heterotrophic sources of soil respiration. Plant Cell Environ 33:1804–19.
Nadelhoffer KJ, Fry B. 1988. Controls on natural nitrogen-15 and carbon-13 abundances in forest soil organic matter. Soil Sci Soc Am J 52:1633–40.
Nickerson N, Risk D. 2009. Physical controls on the isotopic composition of soil-respired CO2. J Geophys Res 114:G01013.
Noy-Meir I. 1973. Desert ecosystems, environments and producers. Ann Rev Ecol Syst 4:25–51.
Ode DJ, Tieszen LL, Lerman JC. 1980. The seasonal contribution of C3 and C4 plant species to primary production in a mixed prairie. Ecology 61:1304–11.
Park R, Epstein S. 1961. Metabolic fractionation of 13C and 12C in plants. Plant Physiol 36:133–8.
Pryor WA. 1973. Permeability-porosity patterns and variations in some Holocene sand bodies. Am Assoc Pet Geol Bull 57:162–89.
Raich JW, Schlesinger WH. 1992. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus 44B:81–99.
Sandford AR, Mott RP, Shuleski PJ, Rinehart EJ, Caravella FJ, Ward RM, Wallace TC. 1977. Geophyscial evidence for a magma body in the vicinity of Socorro, New Mexico. In: Heacock JG, Ed. The earth’s crust: its nature and physical properties, Vol. 20. Washington, DC: American Geophysical Monograph. p 385–404.
Schwinning S, Sala OE. 2004. Hierarchy of responses to resource pulses in arid and semi-arid ecosystems. Oecologia 141:211–20.
Serna-Pérez A, Monger HC, Herrick JE, Murray L. 2006. Carbon dioxide emissions from exhumed petrocalcic horizons. Soil Sci Soc Am J 70:795–805.
Shen W, Jenerette GD, Hui D, Phillips RP, Ren H. 2008. Effects of changing precipitation regimes on dryland soil respiration and C pool dynamics at rainfall event, seasonal and interannual scales. J Geophys Res 113:G03024.
Steinmann K, Siegwolf RTW, Saurer M, Körner C. 2004. Carbon fluxes to the soil in a mature temperate forest assessed by 13C isotope tracing. Oecologia 141:489–501.
Stursova M, Sinsabaugh RL. 2008. Stabilization of oxidative enzymes in desert soil may limit organic matter accumulation. Soil Biol Biochem 40:550–3.
Taylor JA, Lloyd J. 1992. Sources and sinks of atmospheric CO2. Aust J Bot 40:407–18.
Wynn JG, Bird IM, Wong VNL. 2005. Rayleigh distillation and the depth profile of 12C/13C ratios of soil organic carbon from soils of disparate texture in Iron Range National Park, Far North Queensland, Australia. Geochim Cosmochim Acta 69:1961–73.
Wynn JG, Harden JW, Fries TL. 2006. Stable carbon isotope depth profiles and soil organic carbon dynamics in the lower Mississippi Basin. Geoderma 131:89–109.
Acknowledgments
Thanks to the Sevilleta National Wildlife Refuge for providing access, D. Moore for meteorological data, T. Lowrey for identifying plant samples, J. Barnes and J. Dodd for help with sample collection, L. Roberts for assistance with vegetation surveys and to two anonymous reviewers.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
D. Breecker: conceived and designed study, selected study locations, performed research, interpreted results, and wrote article; Z. Sharp: conceived study, helped to write the article, and oversaw stable isotope analyses; L. McFadden: conceived study, helped select study location, and helped to write the article; M. Litvak: interpreted results and helped to write the article; M. Martinez: coded the numerical CO2 model and performed research using the model.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Breecker, D.O., McFadden, L.D., Sharp, Z.D. et al. Deep Autotrophic Soil Respiration in Shrubland and Woodland Ecosystems in Central New Mexico. Ecosystems 15, 83–96 (2012). https://doi.org/10.1007/s10021-011-9495-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-011-9495-x