Abstract
Stroke is one of the leading causes of long-term disability among adults and contributes to major socio-economic burden globally. Stroke frequently results in multifaceted impairments including motor, cognitive and emotion deficits. In recent years, brain–computer interface (BCI)-based therapy has shown promising results for post-stroke motor rehabilitation. In spite of the success received by BCI-based interventions in the motor domain, non-motor impairments are yet to receive similar attention in research and clinical settings. Some preliminary encouraging results in post-stroke cognitive rehabilitation using BCI seem to suggest that it may also hold potential for treating non-motor deficits such as cognitive and emotion impairments. Moreover, past studies have shown an intricate relationship between motor, cognitive and emotion functions which might influence the overall post-stroke rehabilitation outcome. A number of studies highlight the inability of current treatment protocols to account for the implicit interplay between motor, cognitive and emotion functions. This indicates the necessity to explore an all-inclusive treatment plan targeting the synergistic influence of these standalone interventions. This approach may lead to better overall recovery than treating the individual deficits in isolation. In this paper, we review the recent advances in BCI-based post-stroke motor rehabilitation and highlight the potential for the use of BCI systems beyond the motor domain, in particular, in improving cognition and emotion of stroke patients. Building on the current results and findings of studies in individual domains, we next discuss the possibility of a holistic BCI system for motor, cognitive and affect rehabilitation which may synergistically promote restorative neuroplasticity. Such a system would provide an all-encompassing rehabilitation platform, leading to overarching clinical outcomes and transfer of these outcomes to a better quality of living. This is one of the first works to analyse the possibility of targeting cross-domain influence of post-stroke functional recovery enabled by BCI-based rehabilitation.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Stroke is a highly prevalent, life-threatening neuro-vascular emergency. It is the fifth largest cause of death worldwide and is also one of the leading reasons for acquired disabilities in adults[1]. The number of stroke incidents has been projected to be rising steadily with increasingly ageing population. Also, improvements in healthcare technology has resulted in decrease in stroke fatalities leading to a large and increasing number of people living with permanent post-stroke impairments. It is estimated that close to 1% of the world population is living with after-effects of cerebrovascular incidents [1]. Impaired motor control [2], general cognitive deficits [3–5], difficulties in generating or processing speech [6], and altered emotional state [7] are some of the commonly observed debilitating effects of stroke.
With close to 30% of survivors suffering from chronic motor disabilities, hemiplegia or hemiparesis is the most common and the most disabling condition post-stroke [1, 8]. Therefore, the majority of efforts in post-stroke rehabilitation target motor function restoration and there is a growing need for better and efficient rehabilitative interventions. In the last decade, brain-computer interface (BCI) systems have emerged as one of the promising tools for motor function restoration. A BCI system provides a real-time window in decoding of brain dynamics, enabling us to interact with our environment by employing control signals generated solely by brain activity [9]. In the context of motor rehabilitation, BCI systems decode the patients' intention to move their affected limb and these decoded intentions are then used to provide a contingent sensory-motor feedback to the patient in various forms like actual movement, haptic feedback, visual feedback, etc. Recent studies indicate that by bridging the stroke-induced gap between motor intention and sensory feedback of motor movement, BCI-based interventions may lead to functional recovery [10, 11]. Furthermore, growing clinical evidence suggests that BCI may be as effective as some of the best traditional interventions for post-stroke upper limb motor rehabilitation [12].
Besides motor deficits, stroke patients also frequently suffer from cognitive impairments. Various studies have indicated that as many as 25%–80% of the stroke survivors suffer from post-stroke cognitive impairments (PSCI) [21]. The vast majority of rehabilitation of PSCI is done via conventional therapy. Recent studies have demonstrated the efficacy of computer-assisted cognitive rehabilitation (CACR) over traditional methods of cognitive training [22, 23]. CACR-based methods facilitate home-based therapy and reduce the need for an in-person training. Furthermore, BCI-based systems, while being a subtype of CACR systems, have also been shown to renormalize neurophysiological mechanisms of altered cognitive functions in disorders such as attention deficit hyperactive disorder (ADHD) [24, 25]. This renormalized brain activity was seen to correlate with reduced cognitive impairments. Not surprisingly, preliminary results from recent case studies have provided promising evidence for use of BCI-based cognitive training in stroke patients [23, 26]. Further studies are necessary to convincingly demonstrate the efficacy of BCI-based intervention for cognitive rehabilitation in stroke patients.
PSD is another major deficit that has been seen to affect other rehabilitative efforts by reduced patient engagement. In fact depression is only one of the many other psychoemotional deficits faced by stroke survivors. Other emotion-based disorders have also been observed in stroke patients such as general anxiety disorders [18]. In spite of having high prevalence, depressive and other emotion-related deficits typically receive the least amount of attention during post-stroke rehabilitation even though they have been attributed as critical bottlenecks in transferring improved clinical outcomes to quality of living [27]. Many patients do not seem to respond to current treatments for emotion deficits which are mostly based on pharmacotherapy and are known to have adverse side-effect profiles. It has been suggested that close-loop brain stimulation-based techniques monitored in real time using BCIs may thus provide an effective means of treatment for stroke-induced emotional dysfunctions [28].
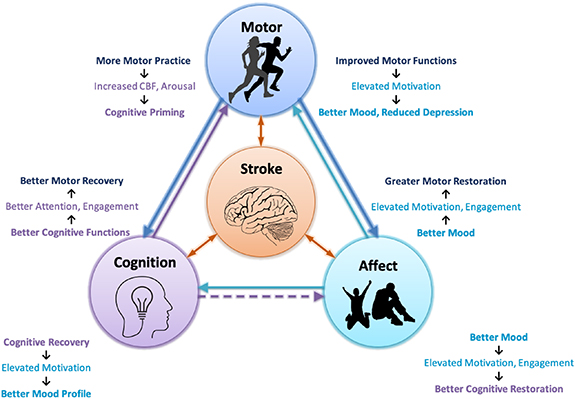
Self-regulation and real-time monitoring of one's mental states thereby provides an important mechanism for re-learning of lost functions due to stroke. Thus, BCIs offer a huge potential to develop an all-encompassing and holistic treatment for stroke. This paper also provides a fresh perspective to BCI-based stroke rehabilitation by specifically targeting the cross-domain influence of functional recovery and neuroplastic changes brought about by neurofeedback training. Figure 1 depicts the various facets of post-stroke deficits. More importantly, it also helps visualize the aforementioned possible interactions between the various domains of stroke induced impairments and how their interrelationships can aid or exacerbate their recovery during post stroke rehab. The aim of this paper is not only to highlight how BCIs can potentially be used to address these various types of impairments but also provide some suggestions on how it may serve as a worthy platform for exploiting the cross-domain influences of the recovery of important neurophysiological functions for a more comprehensive rehabilitation.
Figure 1. Post-stroke motor, cognitive, and affect deficits and the complex interaction between them: Stroke-induced neuronal damage more often than not manifests in varying degrees of motor, cognitive and affect impairments. These impairments, while having standalone debilitating effects, also negatively influence the rehabilitation efforts targeting other domains and recovery of one factor may indirectly benefit the restoration of the other two. Accumulating evidence suggests that improved cognitive functions like attention result in the recovery of upper and lower limb motor control [13, 14], possibly due to better patient engagement. The reverse influence has also been observed wherein aerobic exercises prior to cognitive rehabilitation resulted in increased CBF and arousal which tend to prime the brain for cognitive training [15–17]. Similarly, the affect-related factors like depression and anxiety are known to have a huge negative impact on patients' daily life. Furthermore, studies indicate that patients who are generally treated for post-stroke depression (PSD) tend to have better motor and cognitive rehabilitation outcomes as compared to non-treated patients [18]. Also, better motor rehabilitation has been shown to correlate with motivation and mood profile in post-stroke patients [19, 20]. All these observations demand the exploration of a holistic rehabilitation plan that might achieve more than additive outcomes by synergistic interactions between the motor, cognitive and affect systems.
Download figure:
Standard image High-resolution imageThe remainder of the paper is organized as follows. We first review the recent advances in BCI-based motor rehabilitation. We then present recent findings that indicate the effect of cognitive and affect impairments on motor and overall stroke rehabilitation outcome and highlight the interaction between these major functions. Building on the findings from motor domain, we analyse the feasibility of BCI-based intervention for cognitive and affect rehabilitation. Lastly, we discuss the potential of realising a holistic BCI system for comprehensive motor, cognitive and affect rehabilitation targeting the multifaceted post-stroke impairments.
2. Principles of BCI for rehabilitation
There are primarily two BCI strategies pursued to improve quality of life among stroke impaired patients. The first strategy, named assistive BCI, aims to altogether bypass the damaged neuronal pathways by providing a continuous and permanent alternative for communication and control of external devices [9]. The second strategy, that is called rehabilitative BCI, aims for the recovery of damaged neuronal links and thereby the restoration of impaired functional capabilities by effective facilitation of neuroplasticity [29]. Since functional independence and return to normal life without the need of any supportive devices is desired, rehabilitative BCIs have always been preferred in post-stroke therapy and are the focus of this review.
Neuroplasticity is the brain's ability to undergo substantial structural and functional reorganisation throughout human life and it forms the scientific basis of all the rehabilitative efforts of brain restoration [30, 31]. In fact, numerous neuro-imaging studies have confirmed that the cognitive and motor function improvements following post-stroke rehabilitation are associated with structural modification and functional recovery in the brain [11, 31]. Hence, it is postulated that the effectiveness of any post-stroke rehabilitative intervention will be dependent on its ability to promote restorative neuroplasticity [11].
The stimulation of neuroplasticity from BCI intervention can be conceptualised primarily into four mechanisms; viz, 1. Neurofeedback training, 2. Operant conditioning by reinforcement, 3. Reinforcement of neuronal circuits by repetitive engagement and, 4. Hebbian learning.
Neurofeedback training refers to the volitional/conscious modulation of brain activations by the user [32]. Reduced cortical activity and slowing of brain rhythms in the insulted hemisphere are some of the well-known deficits following stroke and are posited to be associated with motor and cognitive impairments [33, 34]. Neurofeedback training targets these abnormal activations with the philosophy that restoration of brain activations to 'more normal' state will result in functional recovery [10]. To achieve this goal, the patients are provided with a continuous visualization of the brain activity from certain regions and are asked to volitionally up or down regulate this activity. As an example, in multiple motor rehabilitation studies, patients are asked to up-regulate the cortical activity in the mu and beta bands from the lesioned primary or supplementary motor areas to achieve improvements in upper limb motor functions [13, 35–37]. Similar studies have been conducted in the cognitive domain as well, wherein patients aim to increase their attention index which is calculated from EEG such as the beta/theta band power from the prefrontal region as a treatment for ADHD [38, 39]. It has been shown that repetitive neurofeedback training results in a long-term and sustained change in the targeted activation patterns and this change is associated with a reduction in functional impairments [13, 35–37, 40].
Operant conditioning by reinforcement is one of the classical mechanisms of human learning wherein modification in the strength of a behaviour is achieved by rewarding the desired actions and punishing the undesired ones [41]. In the context of BCI, the operant conditioning is achieved by rewarding the patient in a form of visual or sensory feedback upon successful elicitation of the targeted action and providing negative or no feedback on insufficient activations. Most of the BCI systems designed for motor rehabilitation follow this mechanism wherein the successful elicitation of the attempted/imagined limb movement by the patient is rewarded by actual movement of the stroke-affected limb using a robot or electrical stimulation whereas an unsuccessful attempt does not produce any movement [42–46]. This kind of intrinsic, contingent and scheduled reward of success is expected to the drive neuroplastic changes in the same way as the human brain learns to interact with a novel stimulus. Also, repetitive success is sought to drive motivation which in-turn may enhance the rehabilitative efficacy of the BCI intervention [10].
The effectiveness of the neurofeedback training and operant conditioning highly depends on identification of the best cortical activation target to train, which is not a trivial task. Hence, to altogether circumvent this problem, rather than training on a particular rhythm, some BCI systems focus on natural tasks as a whole. As an example, some of the BCI systems for motor rehabilitation train the patients to perform motor movement/imagination [47–52]. The training is achieved by first recording the whole brain activation pattern associated with the specified tasks and then strengthening it by repetitive training wherein identification of this pattern is provided with rewarding feedback. The fact that multiple brain systems are involved in the execution of most of the cognitive, and motor tasks and simultaneous activation, and successful coordination between these systems is necessary for functional improvement forms the basis for this mechanism. Damage to the white matter systems like corpus callosum and corticospinal tract following stroke hampers this essential coordination and results in functional impairments [33, 34, 53, 54]. Hence, the BCI-based, task-focused training that results in repetitive recruitment of the normal motor or cognitive circuits, may strengthen the stroke affected neuronal connectivity leading to functional improvements.
'Neurons that fire together wire together', which is the principle of Hebbian plasticity, is thought to be one of the important neuronal repair mechanisms stimulated by the BCI systems, particularly in the motor rehabilitation settings. Motor impairments following stroke create a gap between motor intention and execution wherein patients' intention to move does not produce any actual movement which results in the lack of sensory feedback to the brain [10, 55]. Some of the BCI systems aim to bridge this gap and close the natural motor loop by providing immediate sensory feedback using robotic or haptic devices that is contingent with users' movement intention [43, 47–52, 56, 57]. Here, it is hypothesised that re-establishing the contingency between cortical activity related to the attempted or imagined movement and the proprioceptive feedback (actual movement) may strengthen the sensory-motor loop and stimulate the neuroplasticity that leads to motor recovery. This simultaneous activation of the outputs and inputs to the motor cortex which triggers the Hebbian plasticity has been thought of as a mechanism behind the neuroplasticity following these rehabilitative interventions [30, 31, 56].
Lastly, the above-mentioned mechanisms are complementary to each other and hence, depending upon the selection of neuronal rehabilitation target and the design of the feedback modality, any rehabilitative BCI system may stimulate the brain recovery by any or all of the mentioned mechanisms.
3. BCI for post-stroke motor rehabilitation
Motor rehabilitation is by far the most researched application of BCI in the stroke domain. In fact, restoration of upper extremity motor impairments in severely impaired stroke patients served as initial motivation for the exploration of BCI technology in the post-stroke rehabilitation field. Patients with severe impairments do not posses the minimum movement capabilities that are necessary to be eligible for the conventional rehabilitation paradigms like occupational therapy (OT) or constrained induced movement therapy (CIMT) and this necessitates the search for a novel rehabilitative intervention. The findings that even the imagination of motor movements; known as motor imagery (MI) results in the recruitment of the same neuronal circuit associated with the actual movement indicated the potential of BCI for rehabilitation. This observation prompted the exploration of whether the severely affected stroke patients who present with complete loss of motor control can perform MI and generate the cortical activations associated with the MI. An initial study comprising eight chronic stroke patients with sever upper limb hemiplegia indicated that most of the patients could indeed successfully modulate the sensory-motor rhythms and control a magnetoencephalography-based BCI system [35]. Although this study did not report any functional improvement, it paved the way for further exploration of rehabilitative potential of BCI technology. The first report on clinical improvements was published by Ang et al [58] wherein eight chronic stroke patients participated in a BCI mediated upper limb rehabilitation. The BCI system was based on the MI protocol and successful detection of MI from real-time EEG signals was rewarded with the centre-out movement of the impaired hand using a MIT-Manus robot. Following 12 rehabilitation sessions over 4 weeks, a significant improvement in motor function of 4.9 points was observed on a Fugl–Meyer Assessment scale. Another longitudinal case study that comprised 60 BCI rehabilitation sessions spread over a period of 8 months indicated that clinical improvements following rehabilitation are associated with the increased cortico-spinal tract integrity and enhanced cortical activations in the sensory-motor area [59].
Following the initial clinical results with support from neuroimaging studies, in the last decade, extensive research has been conducted, exploring the application of BCI for post-stroke motor rehabilitation. Multiple controlled trials have been conducted and they have indicated that, while being available to much diverse spectrum of patients, BCI systems can achieve clinical gains which are at par with conventional rehabilitation techniques like OT, robotics, and functional electric stimulation for upper limb motor restoration following stroke [13, 43, 47, 48, 50, 60–65]. Also, a 2018 meta-analysis of these studies observed that BCI intervention is associated with standardised mean difference (SMD) of 0.79 on upper extremity FMA scale which is comparable with other widely used therapies like CIMT (SMD = 0.81), mirror therapy (SMD = 0.61), and robotics (SMD = 0.35) [12]. Moreover, multiple excellent reviews have provided an update on the state-of-the-art development in rehabilitative BCI field [10, 12, 29, 66, 67] and similarly this section provides a thematic review of motor rehabilitation with a focus on the recent advances and new challenges. For the complete list of BCI-based motor rehabilitation studies in stroke affected patients please refer to tables 1 and 2.
Table 1. Post-stroke BCI-based motor rehabilitation studies.
| Study | Chronicity | BCI Modality | Feedback | N | Sessions | Duration(weeks) (ses/w) | Outcome | FMA Gains |
|---|---|---|---|---|---|---|---|---|
| Upper Limb Rehabilitation : Controlled Studies with non-BCI control | ||||||||
| Mihara et al (2013) [60] | Mixed | NIRS | Visual | 10 | 6 | 2 (3) | UE-FMA, ARAT, MAL, KVIQ | 4.8 |
| Visual-Random | 10 | 2.3 | ||||||
| Pichiorri et al (2015) [61] | Sub-acute | EEG | Visual | 14 | 12 | 4 (3) | UE-FMA, MRC, NIHSS, MAS | 13.6 |
| Visual-Random | 14 | 6.5 | ||||||
| Rayegani et al (2014) [62] | Mixed | EEG | OT, Visual-EEG | 10 | 10 | 2 (5) | JHFT | - |
| OT, Visual-EMG | 10 | - | ||||||
| OT | 10 | - | ||||||
| Ramos-Murguialday et al (2013) [43] | Chronic | EEG | Robotic | 16 | 20 | 4(5) | cFMA, AS, GAS, MAL | 3.4 |
| Robotic-Random | 16 | 0.4 | ||||||
| Ang et al (2014) [47] | Chronic | EEG | Robotic | 6 | 18 | 6 (3) | UE-FMA | 7.2 |
| Robotic-no BCI | 8 | 7.3 | ||||||
| OT | 7 | 4.9 | ||||||
| Ang et al (2015) [50] | Mixed | EEG | Robotic | 11 | 12 | 4 (3) | UE-FMA | 4.5 |
| Robotic-no BCI | 14 | 6.3 | ||||||
| Frolov et al (2017) [63] | Mixed | EEG | Robotic | 55 | 10 | 2 (5) | UE-FMA, ARAT, MAS | 5.0* |
| Robotic-random | 19 | 5.0* | ||||||
| Cheng et al (2020) [109] | Chronic | EEG | Robotic | 5 | 18 | 6 (3) | UE-FMA, ARAT | 3.8 |
| Robotic-no BCI | 5 | 5.6 | ||||||
| Li et al (2014) [48] | Sub-acute | EEG | FES | 8 | 24 | 8 (3) | UE-FMA, ARAT | 12.7 |
| FES-no BCI | 7 | 6.7 | ||||||
| Kim et al (2016) [64] | Mixed | EEG | FES, AOT | 15 | 20 | 4 (5) | UE-FMA, MAL, MBI, Wrist flexion range | 7.9 |
| AOT | 15 | 2.9 | ||||||
| Biasiucci et al (2018) [64] | Chronic | EEG | FES | 14 | 10 | 5 (2) | UE-FMA, MAS, MRC, ESS | 6.6 |
| FES-Random | 13 | 2.0 | ||||||
| Jang et al (2016) [13] | Sub-acute | EEG | FES | 10 | 30 | 6 (5) | MFT, MAS, VAS, Soulder Subflexion | - |
| FES - no BCI | 10 | - | ||||||
| Lower Limb Rehabilitation Studies | ||||||||
| Chung et al (2015) [14] | Chronic | EEG | FES | 5 | 5 | 1 (5) | TUG, BSS, Cadence, Step Length | - |
| FES- no BCI | 5 | - | ||||||
| Lee et al (2015) [91] | Sub-acute | EEG | Visual | 10 | 24 | 8 (3) | 10mWS | - |
| Visual-sham | 10 | - | ||||||
| McCrimmon et al (2015) [44] | Chronic | EEG | FES | 9 | 12 | 4 (3) | LM-FMA, Gait Speed, Dorsiflexion ARMO, 6MWD | 2.4 |
| Mrachacz-Kersting et al (2016) [77] | Mixed | EEG | CPNS | 13 | 3 | 1 (3) | LM-FMA, MRS, NIHSS, AS, 10mWS | 0.8 |
| CPNS - Random | 9 | -2.0 | ||||||
| Tang et al (2018) [92] | Chronic | EEG | Visual | 13 | 12 | 4 (3) | TUG, 10mWS | - |
| Mrachacz-Kersting et al (2019) [93] | Sub-acute | EEG | CPNS | 12 | 12 | 4 (3) | LM-FMA, MRS, NIHSS, AS, 10mWS | 5.9 |
| Sham CPNS | 12 | 2.7 | ||||||
| * : Results are median improvement; Var: Variable, variation range is not specified. | ||||||||
| EEG: Electroencephalography; MEG: Magnetoencephalography; NIRS: Near-Infrared Spectroscopy; FES: Functional Electrical Stimulation; NMES: Neuromuscular Electrical Stimulation; VR: Virtual Reality; OT: Occupational Therapy; AOT: Action Observation Therapy; TS: Tongue Stimulation; rTMS: Repetitive Transcranial Magnetic Stimulation; tDCS: Transcranial direct current stimulation; CPNS: Common Peroneal Nerve Stimulation; BCI: Brain-Computer Interface | ||||||||
| FMA: Fugl- Meyer Assessment; UE-FMA: Upper Extremity part of FMA; cFMA: combined hand and arm scores (motor part) from the modified upper limb FMA; ARAT: Action Research Arm Test ; KVIQ: Kinesthetic and Visual Imagery Questionnaire; MAL: Motor Activity Log-10 ; GAS: Goal Attainment Scale; AS: Ashworth Scale; MAS: Modified AS; McI: Motricity Index; NHPT: Nine Hole Peg Test; GS: Grip Strength; F&M: Fatigue and Mood scale; SIAS: Standardized Measure of Stroke Impairment Scale; MRC: Medical Research Council scale; NIHSS: National Institute of Health Stroke Scale; SIS: Stroke Impact Scale; MFT: Manual Function Test; VAS: Visual Analogue Scale; MBI: Modified Barthel Index; FIM: Functional Independence Measure; TRI-HFT: Toronto Rehabilitation Institute - Hand Function Test; RS: Rankin Scale; MRS: Modified RS; ESS: European Stroke Scale; BBT: Box and Block Test; WMFT: Wolf Motor Function Test; FAT: Frenchay Arm Test of Function; MoCA: Montreal Cognitive Assessment; JHFT: Jebsen Hand Function Test. | ||||||||
| LM-FMA: Leg Motor FMA; ARMO: Active Range of Motion; 6MWD: Six-Minute Walk Distance; TUG: Time Up to Go test; 10mWS: 10 meter walk speed | ||||||||
Table 2. Post-stroke BCI-based motor rehabilitation studies.
| Study | Chronicity | BCI Modality | Feedback | N | Sessions | Duration(weeks) (ses/w) | Outcome | FMA Gains |
|---|---|---|---|---|---|---|---|---|
| Upper Limb Rehabilitation : Controlled Studies with BCI intervention in both groups | ||||||||
| Ono et al (2014) [109] | Mixed | EEG | Visual | 6 | 12–20 | 4 (Var) | SIAS, EMG | - |
| Robotic | 6 | - | ||||||
| Ang et al (2015) [49] | Chronic | EEG | Robotic, tDCS | 10 | 10 | 2 (5) | UE-FMA | 5.0 |
| Robotic, Sham tDCS | 9 | 5.4 | ||||||
| Kasashima-Shindo et al (2015) [85] | Mixed | EEG | Robotic, tDCS | 11 | 10 | 2 (5) | UE-FMA, MAS | 6.0 |
| Robotic | 7 | 6.6 | ||||||
| Johnson et al (2018) [86] | Chronic | EEG | VR, rTMS | 2 | 18 | 6 (3) | BBT | - |
| VR, sham rTMS | 1 | - | ||||||
| Mottaz et al (2018) [110] | Chronic | EEG | Visual -ipsi-M1 | 10 | 16 | 16 (2) | UE-FMA, MAL, GS, 9HPT, BBT, | 5.3 |
| Visual-Control | 10 | MRC,Gait Speed, TUG | 2.0 | |||||
| Upper Limb Rehabilitation: Studies without Control Group | ||||||||
| Ibanez et al (2017) [56] | Chronic | EEG | FES | 4 | 8 | 4 (2) | UE-FMA, SIS | 11.5 |
| Jovanovic et al (2019) [111] | Chronic | EEG | FES | 1 | 80 | 28 (3) | ARAT, UE-FMA, FIM, TRI-HFT | 17.0 |
| Meng et al (2008) [42] | Chronic | EEG | FES | 2 | 20 | 8 (NA) | UE-FMA, ARAT, MAS | - |
| Daly et al (2009) [112] | Chronic | EEG | FES | 1 | 9 | 3 (3) | Index finger extension | - |
| Takahashi et al (2012) [113] | Chronic | EEG | FES | 1 | 2 | - | EMG | - |
| Marquez-Chin et al (2016) [114] | Chronic | EEG | FES, Robotic | 1 | 40 | 14 (3) | UE-FMA, ARAT, FIM, TRI-HFT | 6.0 |
| Nishimoto et al (2018) [115] | Chronic | EEG | FES, Robotic | 26 | 10 | 1.42 (7) | UE-FMA, MAL | 3.3 |
| Zhang et al (2018) [52] | Chronic | EEG | FES, Robotic | 1 | 18 | 6 (3) | WMFT, FMA | 0.0 |
| Mukaino et al (2014) [116] | Chronic | EEG | NMES | 1 | 40 | 8 (5) | UE-FMA, MAS | 7.0 |
| Kawakami et al (2016) [117] | Chronic | EEG | Robotic | 29 | 10 | 2 (5) | UE-FMA, MAS, MAL | 3.3 |
| Sullivan et al (2017) [118] | Chronic | EEG | Robotic | 6 | 12 | 4 (3) | UE-FMA | 3.3 |
| Caria et al (2011) [59] | Chronic | EEG, MEG | Robotic | 1 | 60 | 12 (5) | UE-FMA, WMFT, MAS, GAS | 11.0 |
| Belardinelli et al (2017) [119] | Chronic | EEG | Robotic | 8 | 20 | 4 (5) | UE-FMA | 3.2 |
| Buch et al (2008) [35] | Chronic | MEG | Robotic | 8 | 13–22 | 3–8 (3–5) | BCI accuracy | - |
| Shindo et al (2011) [120] | Chronic | EEG | Robotic | 8 | 12–20 | 16–28 (1–2) | EMG, TMS, SIAS, MAL, MAS | - |
| Bundy et al (2017) [80] | Chronic | EEG | Robotic | 10 | 60 | 12 (5) | ARAT, GS, MAS, RS, McI, AROM | - |
| Darvishi et al (2017) [78] | Chronic | EEG | Robotic | 1 | 10 | 2 (5) | ARAT | - |
| Norman et al (2018) [121] | Chronic | EEG | Robotic | 8 | 12 | 4 (3) | BBT | - |
| Lu et al (2019) [57] | Chronic | EEG | Robotic | 21 | 20 | 6 (Var) | Wrist AROM, BI | - |
| Chowdhury et al (2018) [51] | Chronic | EEG | Robotic | 4 | 12–16 | 6 (2–3) | GS, ARAT, VAS | - |
| Foong et al (2019) [122] | Mixed | EEG | Visual | 11 | 18 | 6 (3) | UE-FMA, ARAT, FAT, GS | 4.4 |
| Mottaz et al (2015) [36] | Chronic | EEG | Visual | 1 | 7 | 4 (2) | UE-FMA, GS, NHPT | 7.0 |
| Prasad et al (2010) [74] | Chronic | EEG | Visual | 5 | 12 | 6 (2) | McI, ARAT, NHPT, GS, F&S | - |
| Song et al (2015) [45] | Mixed | EEG | Visual, FES, TS | 13 | 15 | 6 (2–3) | NIHSS, ARAT, SIS - hand, 9HPT | - |
| Remsik et al (2018) [46] | Mixed | EEG | Visual, FES, TS | 21 | 9-15 | 6 (2–3) | ARAT, SIS, 9HPT, GS | - |
| Vourvopoulos et al (2019) [71] | Chronic | EEG | VR | 4 | 8 | 3 (Var) | UE-FMA, MAS, SIS | 1.3 |
| Vourvopoulos et al (2019) [72] | Chronic | EEG | VR | 1 | 10 | 3 (Var) | UE-FMA, MAS, SIS, MoCA | 9.0 |
Since reinforcement learning and Hebbian plasticity are thought to be important mechanisms of BCI operation, the role and design of feedback which acts as a reward, is very crucial. Studies have explored the clinical efficacy of numerous feedback modalities like visual [60–62], robotic[47, 50, 67–69], functional electrical stimulation (FES) [13, 48, 64], neuromuscular electrical stimulation (NMES) [70], and more recently, virtual reality (VR) [71, 72] with the main aim to provide perceptual and sensory feedback and all these modalities have been observed to elicit motor recovery when used in conjunction with the BCI control. However, how the selection of these modalities and the design of the feedback paradigm affect clinical outcomes is still elusive. For example, in a controlled study [73], the authors reported that sensory feedback of movement may be the crucial element of the BCI-based rehabilitation and visual feedback alone is not sufficient to evoke functional gains. This observation becomes counter-intuitive in light of the clinical gains following multiple studies using neurofeedback based BCIs [36, 61, 74]. These results may be caused by the type of interface used for visual feedback and hence the effect of the presentation technique used for visual feedback needs to be investigated [75]. Moreover, immersive feedback provided by the VR systems may help in enhancing the effect of visual feedback [71, 72]. Furthermore, contingent sensory feedback using robotics, FES or NMES is thought to promote the neuronal repair by stimulating the Hebbian plasticity. However, it is necessary to explore if the similar clinical recovery can be achieved without the afferent sensory feedback. If equivalent gains can be achieved by using only a visual or VR feedback then it can lead to a much more portable, simpler and affordable rehabilitation system that can be used at home which is one of the ultimate goals for the BCI technology.
Contingent and concomitant activation of the cortical areas is essential for the stimulation of Hebbian plasticity. Hence, timing and latency of feedback become important parameters in the BCI studies that provide sensory feedback. It is postulated that following motor intention, the afferent sensory feedback generated by actual movement is responsible for disinhibition of motor cortex and hence the timing of this disinhibition may be an important factor in determining the effectiveness of motor learning [76]. One initial lower limb rehabilitation study also highlights this point where it is observed that only the group that received a common peroneal nerve stimulation (CPNS) timed to reach the brain at the peak negative phase of the MRCP resulted in functional improvements [77]. Also, one recent upper limb rehabilitation study examined the effect of different feedback interval in a robotic rehabilitation study [78]. Although significant functional improvements were observed, conclusive remarks on optimal timing and latency of feedback could not be extracted. Hence, more studies are necessary to identify the optimal feedback duration and standardisation of the same in future BCI systems is essential.
The widespread inclusion of BCI systems in the regular rehabilitation practice necessitates knowledge about how the duration, intensity and frequency of BCI rehabilitation affect the clinical outcome. One initial study indicates that the total number of sessions and BCI intensity (BCI trials/session) and not the frequency of sessions impacts the final clinical outcomes and neurological improvements [79]. However, another study reported that clinical gains on the Action Research Arm Test scale were not dependent on the BCI usage time [80]. Considering the clinical relevance, lack of studies, and contradicting evidence, investigation in this direction is required.
A few BCI-based upper limb rehabilitation studies have explored the addition of non-invasive brain stimulation (NIBS) as adjuvant therapy. NIBS, which can be provided as a transcranial magnetic stimulation or transcranial direct current stimulation (tDCS), is generally administered just prior to a BCI rehabilitation session to modify the cortical activation state [81, 82]. In stroke patients, generally, excitatory NIBS stimulation is applied over the ipsilesional motor cortex (M1) and inhibitory NIBS stimulation is applied over contralesional M1 with an aim to target the reduced ipsilesional M1 activations and increased inter-hemispheric inhibition [83]. The facilitatory state of the brain following NIBS has been thought to have a priming effect that may enhance the effectiveness of the succeeding BCI therapy [81]. Hence, in recent years a few studies have combined the NIBS with BCI-based rehabilitation [49, 84–86] but the additive clinical benefits of NIBS are not clear. Two controlled studies that have used tDCS for brain priming have reported similar clinical outcomes in control and experimental groups [49, 85]. However, despite similar clinical gains, neuroimaging studies indicate that the brain recovery mechanisms stimulated by BCI and BCI+tDCS intervention are very distinct [53, 87, 88]. These findings indicate a potential for sequential training using BCI and BCI+NIBS interventions with a possibility of additive clinical gains.
Also, before proceeding with the inhibitory stimulation of the contralesional hemisphere using NIBS, thorough understanding of the role of the contralesional hemisphere in the post-stroke condition is necessary. It is a general consensus that the contralesional hemisphere shows increased activation following stroke [83]. It is postulated that this increased activation results in an enhanced inhibitory drive on the lesioned hemispheres [83]. Contrarily, a few studies suggest that the increased activation in the contralesional hemisphere is the brain's attempt to compensate for the lost ipsilesional functions [30]. BCI rehabilitation trials have been conducted on the basis of both positive [80, 89] and negative [49, 86] role of the contralesional hemisphere and all these studies have shown clinical improvements. Hence, more knowledge about the role of the contralateral hemisphere is necessary and this may lead to some major modifications in the existing BCI protocol [83].
Lower extremity motor impairments and disturbed gait have a far greater impact on stroke patients' daily lives [90]. Hence, following the success in upper limb rehabilitation, a few studies have explored the applicability of BCI for lower limb motor function restoration. In a controlled trial, Chung et al have reported significant clinical improvements in the timed up and go test (TUG), cadence, and step length following a BCI-FES intervention for five sessions [14]. More importantly, in this study, the control group which received FES for the same duration did not result in significant improvements. Another study that implemented a BCI-based neurofeedback also reported similar conclusions wherein the experimental group showed significant improvements in gait velocity, cadence, stance phase index, forefoot and hindfoot weight whereas only gait velocity was improved in the control group which received a random neurofeedback [91]. Similarly, Tang et al have reported significant improvements in the TUG following MI-BCI rehabilitation with the visual feedback [92]. All these initial studies indicate promising improvements in the lower limb functions following BCI-based rehabilitation [14, 44, 77, 91–93] and much more focused efforts are necessary along this direction. Many important challenges like the deep cortical representation of foot area, distributed control of gait between cortical neurons and central pattern generators [90], and high level of EMG contamination of neurological signals due to associated motor feedback need to be overcome to achieve widespread use of BCI for lower limb motor rehabilitation.
Lastly, upper limb rehabilitation studies indicate that the motor function improvements following BCI rehabilitation may be associated with the accuracy of the BCI system. Many studies have observed a significant correlation between the BCI accuracy and clinical gains [51, 63, 64, 94, 95]. Moreover, most of the controlled trials wherein the control group received random feedback, which is essentially a chance level accurate system, have reported far less functional improvements compared to the experimental group [13, 43, 61, 64]. The reason for this association between BCI accuracy and improvement is not clearly understood. One hypothesis is that higher BCI accuracy may elevate the level of confidence and motivation in patients which may better promote reward-based plasticity. Moreover, higher BCI accuracy may also impart greater patient engagement whereas irrelevant feedback from a less accurate BCI system may result in frustration and hence can negatively affect the rehabilitation [61]. Also, greater engagement may be associated with a higher level of patient attention and this may be one of the reasons behind the success of BCI rehabilitation. This role of attention has been highlighted by one rehabilitation study wherein rehabilitation by attention-based BCI system lead to significant improvements in lower limb motor functions [14]. All these results point towards a complex interaction between motor and various cognitive and affect factors and hence indicate a need for further exploration of the effect of cognition and affect on motor recovery.
4. BCI for post-stroke cognitive training
Besides motor deficits, stroke patients also frequently suffer from cognitive impairments. Cognitive impairments can be seen as a multitude of deficits ranging from inattentive symptoms, slowing of information processing, deficits in memory, deterioration of semantic fluency and difficulties in generating or processing speech or aphasia [96–98]. Speech and language enable complex mental activity such as complex reasoning, forming abstractions and generalisations. Thus, a deficit affecting such critical functions typically has a deep debilitating effect on cognitive functions [98]. Besides cognitive impairments, many of these patients also suffer from other stroke-induced deficits such as motor and PSD. PSCI has been seen to affect the overall rehabilitation outcomes of patients [97]. Most therapies, including BCI-based motor rehabilitation, require a certain minimum cognitive ability of the patient to comprehend and respond to the instructions for carrying out the rehabilitative regimes [47, 50, 60]. Moreover, MI-BCI and other computerised therapies require the patient to maintain sustained attention to the task paradigm for extended periods of time. A patient with severe cognitive impairment who is incapable of meeting such cognitive demands is automatically excluded from rehabilitation and as a result leads a significantly poor quality of life [47, 50, 60]. Therefore, enabling a patient to become acceptable for most regimes of post-stroke rehabilitation could be an important objective for cognitive training.
For patients with moderate to severe PSCI, critical cognitive functions have been seen to improve with traditional methods of post-stroke cognitive training [22]. Surprisingly, while motor functions have typically received a great amount of attention using BCI-based rehabilitation and BCIs have shown great promise in facilitating motor rehabilitation, post-stroke cognitive training using BCIs is still relatively much less explored [99, 100]. Since the effects of BCI-based neurofeedback training have been seen to improve certain cognitive functions in neurodevelopmental and neurodegenerative conditions such as attention-related hyperactive disorder (ADHD) [101, 102] and mild cognitive impairment (MCI) in elderly subjects [103], respectively, it is therefore also likely to generalise to other dysfunctions, including PSCI. Specifically, in previous studies, BCIs have shown promising results for attention training, for instance, in ADHD children, EEG-based neurofeedback therapy showed significant improvement in inattentive and hyperactive-impulsive symptoms [101, 104]. Such results have also been observed by other groups [102, 105]. Besides EEG-based evaluation of brain waves, even neuroimaging-based real-time neurofeedback have recently been shown to provide an effective modality for cognitive training for adolescents with ADHD [106]. Thus, spatially targeted enhancement of cortical regions for self-regulation could be another method which could be used for cognitive training. Encouraging evidence across multiple studies demonstrating the efficacy of neurofeedback-based cognitive training has also been shown by a recent meta-analysis [107]. The authors concluded that neurofeedback training indeed led to significant clinical improvement in reducing ADHD symptoms. Thus, from a behavioural perspective, BCIs have consistently shown to effectively use neurofeedback-training to improve sustained attention.
From a neurophysiological perspective, various observations have been reported that explain the underlying neuromechanisms of neuroplasticity that occur as a result of neurofeedback-based cognitive training. One of the bases for EEG-based neurofeedback training is said to be the re-normalization of EEG patterns that result in improvement of inattentive symptoms [24, 25]. Moreover, various neuroimaging studies have sought to determine the underlying neuromechanisms of clinical improvement following using neurofeedback-training [24, 123, 124]. The fact that neurofeedback-based self-learning can induce such functional changes in the underlying neural properties has important implications for cognitive training for stroke wherein neuroplastic changes have been key to enable repair and restoration of body functions.
A few recent studies have investigated cognitive training in stroke patients using neurofeedback-based treatments. A list of studies using post-stroke neurofeedback training have been listed in table 3. A number of case studies have been reported in literature which have investigated the usability and efficacy of neurofeedback-based cognitive training in single stroke patients [125–128]. For instance, a recent case-study of two chronic stroke patients using neurofeedback-based cognitive training has also shown promising preliminary results [129]. In this study, the intervention was specifically designed to enhance the alpha activity of the patients for both cognitive and motor improvements. Interestingly, the patients also showed improvements in their emotional variables and one of them showed improvements in their speech patterns. This may reflect the ubiquitous functional role of the alpha band for a spectrum of motor and cognitive functions [130–132] and aiming to enhance its activity may be a good strategy to induce a multitude of functional improvements in stroke patients. In fact, neurofeedback strategies aiming at theta inhibition and upper alpha and beta activation have been reported to improve a multitude of cognitive functions as well as the mood profile of the patient [125–128, 133]. This strategy may therefore be extremely valuable for a comprehensive stroke rehabilitation program. In another study, n = 44 stroke patients were randomly allocated to a neurofeedback training (NFB) group, a CACR group and a control (CON) group for cognitive training [23]. Although all groups improved significantly from pre-intervention baseline, the NFB group showed changes in electrophysiological markers after intervention. This observation provides further encouragement to use BCI-based neurofeedback training for cognitive rehabilitation after stroke. More importantly, it reinforces the ability of BCIs to promote neuroplastic changes during training which is quintessential to stroke rehabilitation [25]. Interestingly, a couple other studies have also shown the feasibility of using BCI-based neurofeedback training to improve memory deficits in post-stroke patients [26, 134]. While encouraging results were achieved in [26], the results obtained in [134] were not consistent between the two stroke patients. Therefore, more such studies would be needed to demonstrate the efficacy of BCI systems for improving memory in stroke patients. For more detailed reviews, the reader is suggested to refer to [99, 100, 135].
Given the broad range of cognitive impairments that can result from stroke, some discussion of the relationship between the type of cognitive impairment and the type of BCI-based neurofeedback training used for stroke rehabilitation is warranted. Past studies, including both RCTs as well as case studies, have listed a number of different post stroke cognitive deficits, including inability to maintain sustained attention, deficits in short term, long term and working memory and inability to comprehend or make speech to name a few. A central theme of almost all BCI-based neurofeedback training studies has been to enable self-regulation of particular EEG frequencies that seem to play multifunctional or even fundamental roles in facilitating a wide range of cognitive deficits. It can be found from table 3 that the NFT strategies used across studies have mainly aimed to restrict lower EEG waves such as delta, theta and lower alpha which were found to be abnormally higher in stroke patients before the intervention. This was commonly seen alongside NFT to enhance higher EEG frequencies such as upper alpha and mid-beta. Such similar strategies were seen to improve a number of cognitive (and motor) functions, especially, attention and memory. This could be a result of the ubiquitous role that are attributed to these EEG frequencies and self-regulation of these bands can therefore have the potential to impact a number of different cognitive functions [130, 139]. Moreover, the brain areas used to provide the neurofeedback and enable self-regulation are just as important to note. While some studies used the electrodes that were over the affected brain areas [17, 126], SMR waves were also used in some studies to provide neurofeedback for cognitive functions [26, 128, 134]. An excellent discussion on the role of SMR-based neurofeedback is provided in [26]. Moreover, certain areas such as the frontal cortex have been reported to contain important hubs in attention and cognitive brain networks and have therefore been attributed to be important seats for cognitive functions [140–142]. Therefore, a re-normalizing effect of such brain networks can help improve multiple cognitive functions. However, it is also important to consider that although BCI-based cognitive training has been seen to have an impact on several cognitive functions, a few studies have also alluded to certain specific improvements seen to be enhanced by a particular EEG frequency used for neurofeedback. For instance, in [26], it was also seen that NFT the group using the upper alpha band had more specific improvements in short-term memory compared to the other group that used the SMR waves. The latter had more specific improvements in working memory. Given that there are very few studies which have studied the impact of specific frequencies on the recovery of cognitive functions of patients, it is therefore important to have more such studies in the near future to better understand the interplay between these key factors.
Table 3. Post-stroke BCI-based cognitive rehabilitation studies.
| Study | Study Design | Chronicity | Group | Type of Therapy | Cognitive Deficits | N | Sessions | Duration (weeks) |
|---|---|---|---|---|---|---|---|---|
| Rozelle et al (1995) [125] | CS | Chronic | EEG | AV EEF, followed by EEG NFT | Memory, speech, concentration, anxiety, depression | 1 | 21, 48 | 4-5/week, 22 |
| Bearden et al (2003) [126] | CS | Chronic | EEG | Theta NFT | VM, reading, remembering names, visual deficits | 1 | 42 | 14 |
| Doppelmayr et al (2007) [136] | CC | NA | Study 1: | Memory | 10 , 9-15 | 2 | ||
| EEG | Alpha NFT | 15 | ||||||
| Control | Relaxation training | 17 | ||||||
| Study 2: | ||||||||
| EEG | Alpha NFT | 7 | ||||||
| EEG | Theta NFT | 4 | ||||||
| Control | RSA | 6 | ||||||
| Cannon et al (2010) [127] | CS | Chronic | EEG | NFT | Depression, distractibility, unclear thinking, low motivation, etc | 1 | 52 | 26 |
| Toppi et al (2014) [134] | CS | NA | EEG | SMR NFT | Memory | 2 | 10 | |
| Hofer et al (2014) [137] | CC | Patient | EEG | SMR NFT | Memory | 7 | 10 | 3-5/week |
| Patient | EEG | Theta/Beta NFT | Attention | 6 | 10 | |||
| Healthy | EEG | SMR NFT | 7 | 10 | ||||
| Mroczkowska et al (2014) [128] | CS | Chronic | EEG | SMR NFT | Motor aphasia, anxiety, low motivation, etc | 1 | 30 | 10 |
| Cho et al (2015) [17] | RCT | Chronic | EEG | NFT to enhance SMR & mid-beta, inhibit delta & high-beta | Light cognitive function failure (between 18-23 on MMSE) | 13 | 30 | 6 |
| Control | Traditional occupational and physiotherapy | 14 | ||||||
| Kober et al (2015) [26] | CC | Mixed | EEG | SMR NFT | Memory: ST, LT, WM | 11 | 10 | 3-4 |
| Healthy | EEG | SMR NFT | 16 | |||||
| Chronic | EEG | Upper alpha NFT | 7 | |||||
| Healthy | EEG | Upper alpha NFT | 24 | |||||
| Mixed | Control | TCT | 7 | |||||
| Cho et al (2016) [23] | RCT | Chronic | EEG | NFT to enhance SMR & mid-beta, inhibit delta & high-beta | Light cognitive function failure (between 18-23 on MMSE) | 14 | 2/week | 6 |
| Control | CACR | 14 | ||||||
| Control | TCT | 16 | ||||||
| Kober et al (2017) [138] | CC | Chronic | EEG | Upper alpha NFT | Memory: ST, LT, WM | 2 | up to 10 | 3-5/week |
| Healthy | EEG | Upper alpha NFT | 24 | |||||
| Nan et al (2019) [129] | CS | Chronic | EEG | Alpha NFT | Conduction aphasia, etc | 2 | 15 | 2 months |
| * NA: Information not provided | ||||||||
| Study Design—CS: Case study; CC: Case-Control; RCT: Randomized Control Trial | ||||||||
| Group—EEG: Electroencephalography | ||||||||
| Type of Therapy—AV: Audio-visual; EEF: electroencephalograhic entrainment; NFT: neurofeedback training; RSA: Respiratory sinus arrythmia biofeedback training; SMR: Sensorimotor Rhythm; TCT: Traditional cognitive training; CACR: Computer Assisted Cognitive Rehabilitation; | ||||||||
| Cognitive Deficits - ST: Short-term; LT: Long-term; WM: Working Memory; MMSE: Mini-Mental State Examination | ||||||||
We further note that improvements in cognitive outcomes after stroke may not only be limited to improvements in cognitive functions but also have a simultaneous influence on motor functions and outcomes. A few studies have demonstrated the link between cognitive and motor functions and rehabilitation outcomes [15–17]. For instance, a controlled study using sequential aerobic exercise followed by cognitive training on stroke patients in the intervention group showed significantly improved cognitive outcomes as compared to controls [15]. Motor outcomes of patients improved as well, however without any significant group differences. There may be several reasons enabling this intimate relationship between motor and cognitive functions, especially for the purpose of stroke rehabilitation. Voluntary physical exercise has been reported to cause an increase in the hippocampal brain-derived neurotrophic factor - a protein that is vital to cognitive functioning, especially long-term memory [143]. Therefore, restoration of motor functions may enable a person to better perform physical exercises, which might then help in cognitive repair as well. Moreover, it has been reported that increased cerebral blood flow (CBF) as a result of physical exercise has neuroprotective properties and promotes motor function [144]. This enhanced CBF induced by aerobic exercises may thus act as a priming tool for cognitive training. Aerobic exercises have also been reported to increase arousal levels which may lead to enhanced memory retrieval and cognitive task performance [145]. Combined cognitive and physical exercise training has also been shown to enhance cognitive functioning in the elderly wherein the authors speculated the recruitment of compensatory processes during physical exercise as key to enhancement in cognitive functioning [146].
Given the above observations on the interplay between motor and cognitive functions, we therefore contemplate that combined cognitive and motor training is crucial for a comprehensive stroke treatment. Also, the transfer of improvements in the individual impairment domains may crucially depend on this implicit relationship between the two systems.
5. BCI for post-stroke emotion & mood regulation
Around three decades ago, it had been reported that PSD was a major threat to stroke rehabilitation outcome that could prevent transfer of functional improvement from rehabilitation to activities of daily living [147]. In a recent study, combining sequential aerobic exercises with cognitive training for chronic stroke patients found that even though the patients receiving the sequential treatment improved their functional outcomes significantly over controls, these effects did not transfer to activities of daily living [15]. The authors speculated that among other factors, depression and anxiety—which were not monitored as a part of rehabilitation—could limit this transfer of functional gains to improvement in quality of life. In another study using anti-depressive medication, post-stroke depressive symptoms in n = 100 patients were assessed at multiple times over an 18 month period [148]. There were several important observations from this study. Firstly, a large number of patients developed depressive symptoms during the course of treatment in the first few months following stroke. After a two month period, an association between stroke severity, functional outcome and depressive symptoms started to show, especially as the patients became more aware of their disabilities. Moreover, patients with PSD seemed to show more severe cognitive impairments as compared to non-depressive patients. Another study noted that the development of PSD usually takes a few months after a stroke. Another study on Chinese stroke patients found that about a third of stroke patients also suffered from PSD and came to similar conclusions with regard to observed depressive symptoms and the duration of the course of post-stroke deficits [149]. Moreover, a recent study from Singapore found a negative impact of PSD on upper limb recovery at the end of a 15-week long intervention [150]. These results could have important implications for stroke rehabilitation as it then becomes critical to monitor and treat depressive symptoms in stroke patients, especially in the first few months after stroke. This is also the recommendation of another review study which noted that patients who are generally treated for PSD tend to have better stroke rehabilitation outcomes as compared to non-treated patients [18]. This observation is consistent with other studies although pharmacotherapy is frequently used to treat PSD.
One of the main characteristics of depression is a lack of motivation and action [151]. It has been postulated that depression leads to changes in a person's attitude and a conflict between his belief-desire profile and his actions [151]. That is, for a depressed person, there's a marked difference between what he/she believes they ought to do and what they really do. A lack of motivation (due to depression) leads to a lack of willingness or desire to perform activities that the person knows they ought to perform. This also affects his/her body's response to meaningful stimuli in their environment. Thus, lack of motivation leads to a lack of action which can adversely affect rehabilitation outcome where the patient must actively participate in rehabilitation exercises in order to improve his/her functions. Indeed, this has been observed during a rehabilitation study, wherein loss of interest was shown to be an independent predictor of PSD [152]. Another view that may hold in the context of stroke rehabilitation is a feeling of 'impossibility' [153]. That is, a person may have a desire to participate in rehabilitation exercises and restore his movement but is overcome by the feeling of the goal being too difficult to achieve. This feeling may essentially be exacerbated in severe post-stroke disability. Furthermore, disability-induced depression can be explained through another perspective. Fuchs explains that the body is a medium through which a person perceives the world and reacts according to his set of beliefs and desires [154]. In doing so, however, a person is not aware of all the physical and biochemical processes taking place in his/her body that enable him to perceive and react in a certain way. In severe disability the person becomes extremely conscious of the physically debilitated state of his body which now starts to feel like a burden to his/her own goals and actions.
For BCI-based stroke rehabilitation, encouraging results have recently been obtained which suggest that BCI-based interventions may hold the key to penetrate the lack of motivation felt by stroke survivors due to PSD. A few studies have investigated techniques for uplifting the mood profile of stroke patients and their effects on functional outcomes during post-stroke rehabilitation. For instance, a recent study on n = 65 stroke patients analysed the effect of music on uplifting the mood profile of stroke patients undergoing motor and cognitive rehabilitation [20]. Not only did the music-based therapy find improved mood profile of participants, the group receiving music-therapy also showed better functional outcomes compared to controls who received conventional therapy without music. Another study on a smaller sample of n = 8 of stroke subjects, the BCI performance of upper limb MI was found to be correlated with interest and motivation [19]. Thus, BCIs may be used as a tool to regain motivation and willingness to actively participate in rehabilitation which is necessary to improve motor and/or cognitive functions. Since MI-based paradigms can be used even by patients whose motor functions are severely affected, in theory, encouraging BCI performance using a powerful BCI decoder could act as a stimulant to boost a subject's mood profile and motivation. In recent years, NIBS-based systems have been suggested to further condition the brain for greater improvement in the mood profile of the patient [28]. Another recent and interesting prospect for emotion regulation has been in the integration of music therapy along with BCIs which has shown promising results [155]. Furthermore, in recent years, real-time neuroimaging based neurofeedback training has also shown the ability to self-regulate emotions by targeting the activation of the amygdala [156]. Since amygdala is a deeper lying brain structure, its influence on EEG activity is not well understood. However, using simultaneous neuroimaging and EEG recordings, activation of the amygdala has been seen to be correlated with EEG-based biomarkers [157] which could further help the development of EEG-based BCI systems for emotion regulation. Thus, there is plenty of scope for BCIs to be used for emotion and affect training especially for stroke patients.
6. Future prospects: holistic BCI intervention
In the above sections, we reviewed literature on present standalone practices in stroke rehabilitation in motor, cognitive and affect domains. We now analyse the feasibility of a comprehensive treatment for stroke using BCIs. We argue that there is evidence in literature that encourage further investigation of a combined and holistic treatment of stroke-induced motor, cognitive and affect-related deficits. It is hoped that such an all-inclusive treatment would result in over-arching outcomes, benefiting from the synergistic improvements beyond the effect of individual treatments put together. We next offer some suggestions on how such a comprehensive treatment may be best developed using BCIs.
In the sections above, we have seen that motor and cognitive functions have an implicit relationship that affect the outcome of stroke rehabilitation. Aerobic exercises prior to cognitive rehabilitation help in increasing CBF and arousal which tend to prime the brain for cognitive training outcomes. Evidence of the reverse influence can also be seen when increased cognitive functions such as increased attention led to increased motor gains by increasing patient engagement [13, 14]. Thus, it is reasonable to expect that functional improvements in motor and cognitive training would aid the improvement of the other function. Therefore, instead of separate cognitive and motor rehabilitation, we suggest that future rehabilitation regimes should include motor and cognitive training sessions and closely monitor the improvement in both functions. However, as we have also noted earlier, the type of cognitive deficits in stroke patients seem to occupy a diverse range of functions. While a few studies have noted that NFT helped improve a multitude of different cognitive functions by targeting EEG waves that serve important roles in information processing, very little attention has been paid to the interplay between the type of cognitive and motor deficit and their subsequent recovery. In this regard, there are a few key areas that can be addressed in future studies. For instance, the ability to maintain sustained attention is important to follow instructions during rehab which can majorly affect post-stroke motor recovery. Moreover, behavioural changes that occur alongside improvement in important cognitive functions can influence motor recovery during rehab. Overall, we note that it is important to also study the relationship between the types of cognitive and motor deficits which is currently not well explored in literature and possibly incorporate them as important factors in designing future holistic rehab trials.
Next, we focus on the influence of the mood profile and motivation of a patient with their rehabilitation outcomes of both motor and cognitive training. BCI performance has been seen to be correlated with motivation of stroke subjects. Given the implicit link between motivation and mood profile, increased motivation may help in increasing the patient engagement during rehabilitation. This may then result in better post-stroke rehabilitation outcomes. The reverse influence has also been suggested in literature wherein patients with better mood profile showed better stroke rehabilitation outcomes. Furthermore, when patients were specifically treated for depressive treatments using pharmacotherapy, the intervention group showed better rehabilitation outcomes than the control group which did not receive such treatment. Therefore, we hypothesise that monitoring and treating affect-based disorders within the rehabilitation program may have a significant influence on standard stroke rehabilitation practices. As mentioned earlier, NIBS techniques along with BCIs may provide an effective mechanism to improve the mood-profile of patients during rehabilitation which may then translate to better overall outcomes.
On the basis of the above arguments and hypotheses, we further postulate that BCIs would be the most effective modality to integrate the rehabilitation of various functions together into a single comprehensive treatment. Stroke-induced deficits and the consequent improvements are seen as a result of neuroplasticity and BCIs have demonstrated a tremendous ability to promote neuroplastic changes using neurofeedback-based training. Therefore, BCIs can serve as a common platform to simultaneously target the multifaceted deficits of stroke by invoking the implicit relationship between the motor, cognitive and affect functions.
The use of a holistic BCI system should also be accompanied with a neurophysiology-guided personalised rehabilitation plan. The heterogeneity in the rehabilitation outcomes among stroke patients indicates that the present rehabilitation practice of 'one-fits-all' may not result in best possible recovery for every individual [10, 31, 158]. Therefore, every patient should first be profiled using an all-inclusive battery of clinical and neurophysiological assessments. Then a personalised rehabilitation program should be designed based on the functional needs and neurological biomarkers for every patient [10, 33]. This has also been illustrated in figure 2, which is a visual representation of such comprehensive profiling for a holistic stroke rehabilitation paradigm. As shown in the figure, among the motor, cognitive and affect deficits, the rehabilitation of the most-impaired functions should be individually prioritised to reduce the negative impact of that impairment on rehabilitation therapies targeted for improving the other two. Moreover, the type, dosage and frequency of the rehabilitation sessions should be customised based on the patients' needs and capabilities [80]. Also, a continuous functional assessments should be performed during the rehabilitation program to assess the effectiveness of the prescribed intervention which may then be altered on reaching an intermediate recovery plateau.
Figure 2. Conceptualisation of the comprehensive and personalised program for post-stroke motor, cognitive, and affect rehabilitation: In a perfect scenario, every stroke patient should receive a comprehensive rehabilitation simultaneously targeting the motor, cognitive and affect impairments. At the admission, the patient should be evaluated with an all-inclusive battery of clinical and neurophysiological assessments. With the information of neurophysiological biomarkers, and the severity of impairments, the type, intensity, and duration of the individual rehabilitation should be personalised according to the needs of each patient. Continuous follow-up assessments should be administered to assess the effectiveness of the given intervention which may then be altered on reaching an intermediate recovery plateau. This kind of longitudinal holistic program with neurophysiology-guided fine tuning may help patients to achieve the best possible recovery.
Download figure:
Standard image High-resolution imageBesides the above prospects for future studies for a BCI-based holistic treatment, it also important to note a number of limitations in current studies that need to be addressed. For instance, neurofeedback plays a very important role in BCI-based rehabilitation and a number of factors like feedback timing and modality are key in determining the effectiveness of its delivery to the patient. Yet, few studies have addressed this issue. It is possible that this relatively challenging aspect may be a critical bottleneck that could also benefit by further fine-tuning, personalization and/or adaptation. Another important area which deserves attention is the calibration paradigms used to collect data for building detection models which are typically known to suffer from low subject engagement. Moreover, just like more traditional pharmacological treatments, factors such as treatment (dosage) frequency and intensity also need to be studied in greater detail in order to further enhance the effectiveness of BCI-based treatments. Furthermore, many studies typically have small sample sizes, differences in time of intervention post-stroke and type of controls. These factors make it very difficult to compare and draw conclusions across studies, thus limiting a true holistic understanding of the effects of BCI-based stroke rehabilitation. These are only a few of the various limitations faced by current studies that should be addressed in the near future.
In conclusion, we propose that a BCI based holistic, personalized, longitudinal rehabilitation plan which targets motor, cognitive and affect deficits all at once may be the future of post-stroke rehabilitation.
7. Conclusions
In this paper, we present a non-exhaustive review of recent studies on stroke rehabilitation specifically in motor, cognitive and affect domains. BCI-based rehabilitation has been most widely used to address motor impairments after stroke and have shown to outperform most conventional forms of treatment. Post-stroke cognitive training using BCI has also shown encouraging results. Furthermore, self-regulation of brain waves has been seen to influence the mood-profile of stroke patients. Neuroimaging evidence for functional and structural changes accompanying clinical improvements following BCI-based rehabilitation suggest that BCI-based intervention promote restorative neuroplasticity. More importantly, the cross-modal influence of functional recovery has been observed across motor, cognitive and affect domains. Therefore, building on the evidence accumulated, we propose a holistic, all-inclusive treatment for stroke targeting motor, cognitive and affect functions. Considering that all the stroke-induced impairments originate from the brain, a BCI-centric approach may thus be the most appropriate and feasible modality to carry out such a holistic rehabilitation program. Furthermore, we recommend that an all-inclusive battery of clinical and neurophysiological assessments be carried out to comprehensively profile and develop a personalized rehabilitation program.