Abstract
Support materials are very crucial in noble metal electrocatalyst synthesis. They improve the catalytic activity of the noble metal by increasing their conductivity, surface area, and interactions. This report investigates the effect of support material on palladium nanoparticles' electrochemical activity towards hydrogen evolution reaction. The structural and morphological study was conducted using x-ray diffraction (XRD), Raman Spectroscopy, and Field Emission Scanning Electron Microscope (FE-SEM) that confirmed the support material has a significant effect on the structure of nanocomposite. The hydrogen evolution (HER) performance of the synthesized electrocatalyst was evaluated in 0.5 M H2SO4. The Pd-Ni/g-C3N4 has higher catalytic activity with a lower overpotential of 55 mV at 10 mA cm2 current density and Tafel slope value 56 mV.dec−1 than other support material studied. The overpotential at 10 mA cm2 and Tafel slope value for electrocatalyst studied respectively are:- Pd/MoS2/CB( 78 mV at 10 mA cm2 and 57 mV.dec−1), Pd/g-C3N4(105 mV at 10 mA cm2 and 69 mV.dec−1) and Pd/CB(117 mV at 10 mA cm2 and 68 mV.dec−1). The impedance spectroscopy study shows Pd-Ni/g-C3N4 demonstrated the smallest semicircle. Further, the Chronoamparometry(CP) and linear sweep voltammetry (LSV) stability study of the highest performing electrocatalyst demonstrates negligible loss in current density for 12 h and minor change in the polarization curve after10,000 cycles. This study shows how the support material influences noble metal catalysts' activity and stability via the support- metal interactions.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
Nanomaterials are used for various applications such as energy conversion, storage, and catalysis, as they modify the morphology, structure, and optoelectrical properties of the materials [1, 2]. Catalysts based on nanoparticles/nanocomposites demonstrate higher catalytic activity because of their morphology and nano dimensional character [3].
Support materials help the dispersion of noble metal nanoparticles and reduce the amount of noble metal utilization, thereby reducing the cost. The significant role of support materials in a noble metal catalyst becomes a significant research area because of its application in fuel cells [4, 5]. The support material should possess the following four characteristics (1) good electrical conductivity, (2) provide a higher surface area, (3) have mesoporous structure, and (4) have good interaction(metal-support) [6, 7]. Carbon support materials are widespread for different Pd-based catalysts in various applications. The emergence of graphene opens up a new way of utilizing carbon as two-dimensional support with high conductivity. Fu et al [8] use a surfactant-free method to synthesize Pd/GN and demonstrate higher catalytic activity towards formic acid oxidation resulting from the higher dispersion of PdNP graphene surface.
The carbon-based support materials such as graphene oxide (GO), multi-wall carbon nanotube (MWCN), carbon black (CB), graphitic carbon nitride (g-C3N4) are widespread and mostly used as support material in many catalytic processes [9]. Graphene-based materials are currently used for different applications because of their high specific surface area with good chemical and mechanical stability [10–12]. They also help for increasing electron transfer properties and improve the mass transfer of reactants. The following graphene characteristics make it a better alternative among other types of carbon support: -higher specific surface area, excellent conductivity, higher chemical stability, and low manufacturing cost. However, the Van der Waals interaction that crate restacking in graphene sheet is the main problem. It can be solved by introducing electron-rich species like nitrogen or sulfur.
The graphitic carbon nitride is recently attracting attention among the carbon support materials as alternative support in various applications because of its rich surface properties. It consists of a C-N bond with non-localization of π electron (conjugated polymer) [13–15]. It is also very stable both thermally up to 600 °C in air and hydrothermally (insoluble either in acidic or basic medium) [16] that make the graphitic carbon nitride material a potential candidate as a catalyst and support material. Carbon black Vulcan XC-72 is another most commonly used carbon material as support, especially in polymeric electrolyte fuel cells [17]. It has a high specific surface area of around 250 m2 g−1.
Non-carbon supports such as oxides, carbides, and nitride have also demonstrated promising corrosion resistance under fuel cell conditions [18]. Among non-carbon support materials. Molybdenum disulfide (MoS2) demonstrates improved electrocatalytic activity related to sulfur atom vacancy created at the edge (Mo-S) [19–21].
The study on the effect of support material is recently getting more attention [22, 23]. It refers to the interaction between heterogeneous metal nanocatalyst and the support substrate. The support effect includes morphology (size and shape) [24] disparity [25] stability of nanoparticles [26]. If there are more defects and holes on the surface, there is a strong binding site for anchoring metal nanoparticles [27].
The 'electronic metal-support' interaction indicates the distribution of charge between the metal and the support in a catalytic reaction [28, 29], and it has a significant effect on the catalytic activity.
The metal support interaction was reported for the first time by Tauster et al. They have demonstrated the chemisorption phenomenon that improves the catalytic activity of titanium oxide (TiO2) supported group VIII metals [30]. Among the metal oxides, CeO2 attracts the highest attention because of its high concentration of oxygen vacancies and the strong metal-support interaction properties [31, 32].
Even though there are many reports on the strong metal-support interactions, it is rarely reported for HER [33, 34]. In addition to this, there is ongoing debate concerning the active sites [35]. Recently we have reported Pd/MoS2/N,S-rGO [36] and M(M = Ni, Co, Cu)@Pd/N,S-rGO [37] synthesized by hydrothermal method followed by chemical reduction method. They have demonstrated higher electrocatalytic activity towards HER and OER due to the synergetic effect between PdNP and the supports. In this particular work, we synthesize Pd-g-C3N4, Pd-Ni-g-C3N4, Pd-MoS2/CB, and Pd/CB by hydrothermal wet chemical reduction method and characterized their electrocatalytic activities towards hydrogen evolution reaction (HER). The electrocatalytic activities of these catalysts are compared against our previous reported catalysts.
Experiment
Reagents
All the reagents and chemicals used for this study were analytical grade and used without additional treatment. Nickelnitratehexahydrate(Ni(NO3)2.6H2O)98%, Ethyleneglycol (C2H4(OH)2, 99.8%),N,N-Dimethylformamide(DMF),Thiourea (N2H4SC)(99%) and sodium Molybdate dehydrate(Na2MoO4.2H2O)(99.5%) were purchased from SRL, India, Palladium chloride (PdCl2, 98%, Sigma-Aldrich), Ethanol (98%), Hydrogen peroxide (H2O2, ≥30%), Melamine(C3N6H6) and carbon black(Vulcan XC-72R) were obtained as a gift from Merck. De-ionized water (using Labaqua Bio ultrapure di-ionizer) was utilized for all sample preparation.
Method
Graphitic carbon nitride (g-C3N4) Synthesis
Graphitic carbon nitride (g-C3N4) was synthesized from Melamine. Briefly, Melamine (8 g) heated in a tubular furnace at 550 °C for 3 h, followed by exfoliation of the resulted bulk g-C3N4 used to make a nanosheet of g-C3N4, which is done by ultrasonication. Briefly, bulk g-C3N4 (150 mg) dispersed in deionized water (50 ml) followed by sonication for 8 h at a bath temperature of 28 °C. Finally, the white suspension was obtained.
Pd-Ni/g-C3N4 and Pd/MoS2/CB synthesis
Pd-Ni/g-C3N4 synthesized by reduction of the metal nanoparticles using modified polyol method. Briefly, 40 mg (g-C3N4) dispersed in a solvent of 7 ml 2-propanol and 3 ml of water, followed by sonication for 2 h. PdCl2 (15 mg) and 3 mg NiCl2.6H2O dissolved in water followed by sonication for 1 h. The whole solution is mixed under stirring. The solution's pH was adjusted to 10 by using a solution of 0.1 M NaOH followed by 12 ml of 1 M NaBH4 solution dropwise. Finally, the product was collected by filtration, washing with water, and dried at 120 °C.
Pd/MoS2/CB synthesis
MoS2/CB was synthesized by the solvothermal process (modification of hydrothermal process). Accordingly, 24.2 mg (CB) dispersed in 35 ml (DMF) followed by sonication at room temperature. A 121 mg (0.5 mmol) Na2MnO4.2H2O was added to the above solution, followed by sonication for 20 min. Then 228.0 mg (3 mmol) thiourea was added to the mixture. The whole solution was sonicated for 15 min and transferred to autoclave heated at 220 °C for 24 h. The final product was collected by filtration, washing with deionized water and ethanol dried at 70 °C for 24 h.
Physicochemical characterization
The physicochemical characterization was done using x-ray diffraction (XRD) to study the crystalline nature of the synthesized sample by Shimadzu powder XRD-600 with Cu kαradiation. The Raman spectroscopy (Horiba-Jobin, Model: - LabRAMHR) with Ar laser source 633 nm was used to investigate the synthesized sample defect. A Field Emission Scanning Electron microscope (FESEM) (FEI, Quanta 200) was used to study the surface morphology.
Electrode fabrication
Glassy carbon electrode (geometric area of 0.0706 cm2) was polished and electro conditioned, as reported in our previous work. According to our previous method, the glassy carbon electrode was modified by the drop cast method [36]. Briefly, 5 mg of electrocatalyst mixed [water/ethanol(1:1)] and 10 μl 5% Nafion solution as binder followed by sonication for 30 min. 5 μl of the link is drop cast on the glassy carbon electrode. The loading of palladium on glassy carbon was estimated to be 0.14 mg cm−2.
Measurement of electrochemical activity
The electrochemical activity and stability of the synthesized electrocatalyst towards hydrogen evolution reaction(HER) were evaluated using linear sweep voltammetry(LSV) and Chronoamparometry(CP) in 0.5 M H2SO4. The charge transfer properties of the electrocatalyst were studied using electrochemical impedance spectroscopy (EIS) measurement within frequency range[100 kHz to 0.01 HZ] using a potentiostat (Biologic SP-300) with software EC-Lab V11.10. For all electrochemical activity measurements, a three-electrode configuration was used. Glassy carbon modified electrode used as working electrode, reference electrode Ag/AgCl (saturated KCl), and graphite counter electrode. All the potential in this report converted to reversible hydrogen electrode by the following formula
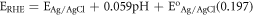
Result and discussion
The crystalline nature of the synthesized sample was studied using x-ray diffraction (XRD) and displayed in figure 1(a). The graphitic carbon nitride supported palladium shows a relatively lower degree of diffraction that could indicate higher palladium nanoparticles' dispersion. The lowest degree was observed in the Pd-Ni/G-C3N4 composite (Pd111 = 39.46, Pd200 = 46.02, and Pd220 = 67.21) related to the strain effect resulted from strong bond formation between Pd-Ni. The Pd/MoS2/CB demonstrate lower degree (Pd111 = 40.11, Pd200 = 46.50 and Pd220 = 67.10) relative to Pd/CB(Pd111 = 40.38, Pd200 = 47.02 and Pd220 = 67.92) that shows the higher dispersion of palladium in Pd/MoS2/CB. The introduction of MoS2 resulted in a synergetic effect that could be a reason for the observed higher dispersion.
Figure 1. (a) XRD pattern for the catalysts Pd-Ni/g-C3N4, Pd-MoS2/CB, Pd/g-C3N4, Pd/CB, MoS2/CB, and Ni/g-C3N4, respectively. Figure 1(b) the Raman shift spectra for the catalysts Pd-Ni/g-C3N4, Pd-MoS2/CB, Pd/g-C3N4, Pd/CB, MoS2/CB, and Ni-g-C3N4, respectively.
Download figure:
Standard image High-resolution imageThe Raman spectroscopy is a vital means to know the degree of defect in the synthesized sample in a non-distractive way. The Raman spectra of the synthesized samples are displayed in figure 1(b). Due to the polymeric nature of graphitic carbon nitride (g-C3N4), the palladium peaks supported on graphitic carbon nitride (g-C3N4) were not sharp and weak. The two common peaks in Raman spectra are the D-band related to the vibrational mode of A1g and G-band related to E2g resulting from starching of SP2 carbon. The ID/IG indicates the degree of defect:- the higher ID/IG ratio shows more defect. Accordingly, comparing Pd-Ni/C3N4 and Pd/MoS2/CB, as shown in table 1, the Pd-Ni/C3N4 demonstrated higher D-band (1478), G-band (1603), and ID/IG (0.922) than Pd/MoS2/CB, which demonstrate D-band (1342), G-band (1595) and ID/IG (0.844) due to the support effect. Similarly, comparing Pd/CB and Pd/g-C3N4, the latter demonstrated a higher D-band (1470), G-band (1601), and ID/IG (0.918) than the former as summarized in table 1.
Table 1. Raman parameter for the synthesized electrocatalyst.
| Electrocatalyst | D-band | G-band | ID/IG |
|---|---|---|---|
| Ni/g-C3N4 | 1468 | 1600 | 0.917 |
| MoS2/CB | 1340 | 1592 | 0.841 |
| Pd/CB | 1343 | 1594 | 0.843 |
| Pd/g-C3N4 | 1470 | 1601 | 0.918 |
| Pd/MoS2/CB | 1342 | 1595 | 0.844 |
| Pd-Ni/g-C3N4 | 1478 | 1603 | 0.922 |
The FE-SEM image of Pd/MoS2/CB shown in figures 2(a) and (b) shows some agglomeration, and figures 2(c) and (d) image of Pd-Ni/g-C3N4 reveals a nanoflake morphology with a clear distribution. The atomic percent of palladium in both composites is almost the same, as indicated in figures 2(g) and (h). This confirms the difference in performance due to the difference in support materials.
Figure 2. FESEM analysis result of Pd-MoS2-CB (a) 500 nm, (b) 5 μm and Pd-Ni-g-C3N4 (c) EDX and (d) corresponding elemental ratio table.
Download figure:
Standard image High-resolution imageHydrogen evolution activity of electrocatalyst
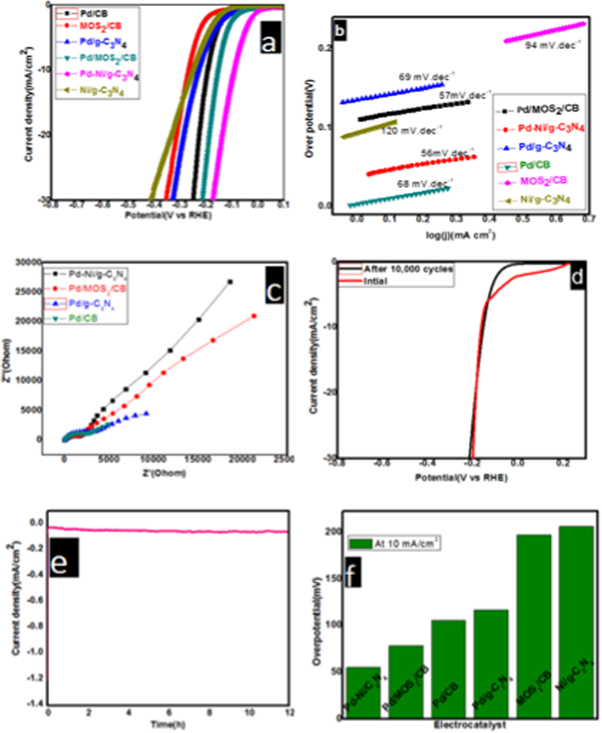
The HER activity of the synthesized electrocatalysts was evaluated using linear sweep voltammetry (LSV) techniques conducted in 0.5 M H2SO4 aqueous solutions with a scan rate of 5 mV s−1 (figure 3). Among the synthesized nanocomposites, Pd-Ni/g-C3N4 demonstrates an overpotential of 55 mV to a chive 10 mA cm−2 current density, which was the lowest compared with the others synthesized nanocomposite in this report, i.e., Pd/MoS2/CB(78 mV), Pd/g-C3N4(105 mV), and Pd/CB (117 mV).
Figure 3. (a) HER polarization curves for Pd-Ni/g-C3N4, Pd-MoS2/CB, Pd/g-C3N4, Pd/CB, MoS2/CB, and Ni-g-C3N4 in 0.5 M H2SO4 with scan rate 5 mVs−1. (b) The corresponding Tafel plot derived from the polarization curve, (c) electrochemical impedance spectroscopy (d), and (e) are the stability test of Pd-Ni/g-C3N4 by Linear Sweep Voltammetry (LSV) and chronoamperometry respectively of the prepared catalysts. (f) Comparison of overpotential at 10 mA for the prepared catalysts.
Download figure:
Standard image High-resolution imageHydrogen evolution starts at the standard potential of zero overpotential Versus RHE but practically higher overpotential is required to push hydrogen evolution at the target electrode. This extra potential is called overpotential. Comparison of overpotential at benchmark current density 10 mA.cm2 was displayed in the bar graph of figure 3(f).
The Tafel slope value indicates the intrinsic behavior of the electrocatalyst. It is also used to propose the reaction mechanism. The Tafel slope values theoretically estimated 120, 40, and 30 mV/decade are associated with Volmer reaction, Heyrovsky reaction, and Tafel reaction, respectively. Accordingly, all the electrocatalysts follow Volumer-Heyrovsky, which involves hydrogen adsorption and desorption in the rate-determining step. Among the synthesized electrocatalysts, Pd-Ni/g-C3N4 demonstrates a lower Tafel slope value (56 mV dec−1) compared to Pd/MoS2/CB(57 mV dec−1), Pd/g-C3N4(69 mV dec−1), and Pd/CB (68 mV dec−1), which indicate the highest activity of the electrocatalyst.
The electrochemical impedance spectroscopy was used to evaluate the electrode polarization. The impedance spectra observed at lower frequencies indicate the material behavior and at a higher frequency associated with bulk electrolyte behavior. Accordingly, in this work, the smallest semicircle demonstrated by Pd-Ni/g-C3N4 indicates the sample's conductive nature.
Stability is an essential parameter for the practical application of the synthesized electrocatalyst. The two common methods by which we study stability measures the variation in current Versus time (I-t curve) and by conducting CV cycles. We use both methods in this particular report to evaluate stability for the best performing electrocatalyst. As displayed in figures 3(d) and (e). Excellent stability with negligible loss in current density for 12 h in Chronoamparometry(CP) curve and minor change of polarization curve of linear sweep voltammetry (LSV) after 10,000 cycles.The hydrogen evolution performance of the as-prepared palladium nanoparticles on a different support material in 0.5 M H2SO4 at 25 oC compared to other reported electrocatalyst is summarized in table 2.
Table 2. Comparison table for hydrogen evolution performance of palladium nanoparticles on a different support material in 0.5 M H2SO4 at 25 °C.
| Electrocatalyst | Onset potential(mv) | Over potential(mv) | Tafel slope (mv.dec−1) | References |
|---|---|---|---|---|
| Pd-Ni/C3N4 | 22 | 55 | 56 | This work |
| Pd/MoS2/CB | 23 | 78 | 57 | This work |
| Pd/g-C3N4 | 56 | 105 | 68 | This work |
| Pd/CB | 44 | 117 | 69 | This work |
| Pd/MoS2/N,S-rGO | 10 | 42 | 38 | [36] |
| Co@Pd/N,S-rGO | 94 | 58 | 54 | [37] |
| Ni@Pd/N,S-rGO | 22 | 35 | 36 | [37] |
| Cu@Pd/N,S-rGO | 42 | 48 | 46 | [37] |
| Ag@PdAg | 38 | 93 | 83 | [38] |
| Pd-CoCNT | 24 | 112 | 56 | [39] |
| Pd27Cu73/C | — | 331 | 126 | [40] |
Conclusion
Different support materials for Pd nanocomposite (CB, g-C3N4, MoS2/CB, and Ni/g-C3N4) were synthesized and characterized using XRD, Raman, FE-SEM, and their electrochemical performance towards hydrogen evolution reaction was evaluated. The synthesized electrocatalyst demonstrated the effect of support material on the electrocatalytic performance of palladium nanoparticles. The graphitic carbon nitride supported palladium shows relatively better dispersion of palladium nanoparticles from XRD and Raman data due to the rich surface properties of g-C3N4. Among the synthesized electrocatalyst, Pd-Ni/g-C3N4 demonstrate the highest hydrogen evaluation performance relative to the other electrocatalyst. Due to the synergetic effect between strong interaction between Pd-Ni and g-C3N4 support. This study demonstrated how we could affect a noble metal catalyst's properties by changing the support materials.